Start Date: September 2013
PDF version
Investigator
Hongjie Dai, Department of Chemistry, Stanford University
Objective
The goal of this work is to design photo-electrochemically rechargeable zinc (Zn)-air batteries using a new concept that integrates metal-air batteries with solar energy harvesting to charge batteries for grid or distributed energy storage at above 80% efficiency. Researchers will develop novel air electrocatalysts with high-efficiency, durable nanocarbon-inorganic hybrid materials for oxygen reduction and/or evolution. The surface engineering of active and stable semiconductor photoanodes will enable the recharging of the photo-electrochemical (PEC) Zn-air batteries under sunlight.
Background
Traditional low-cost aqueous batteries, such as lead acid and nickel-metal hydride, are limited by low energy density. Lithium (Li)-ion batteries offer higher energy density, but are more costly and less safe. Metal-air batteries, such as Li-air, aluminium-air, Zn-air and iron-air, are capable of significantly higher energy densities. Among them, Zn-air is technically and economically the most viable option. These batteries have high energy density (2 to 3 times higher than Li-ion) but limited power density. They are safe, earth-abundant, non-toxic and could be manufactured at low costs.
Zn-air batteries produce electricity through a redox reaction between Zn metal and atmospheric oxygen (O2) in an alkaline electrolyte with ZnO (or soluble zincate, Zn(OH)42-) as the discharge product (Figure 1). In reverse, the O2 and Zn metal are regenerated at the cathode and anode respectively. Their high energy density comes from the fact that the oxygen cathode is not stored in the cell. Instead, a porous air cathode continuously takes in O2 from the air during discharge and exhales O2 upon recharge. The development of rechargeable Zn-air batteries has been mainly hampered by the lack of efficient and robust air-cathode electrocatalysts for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) in the alkaline electrolytes, causing low energy efficiency and cell-cycling stability; and 2) the short lifetime of Zn electrodes, as well as other issues. such as cell design, electrolyte composition and proper carbon dioxide management.
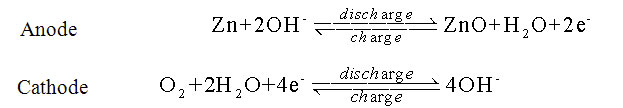
Figure 1: Chemical equations at the electrodes for Zn-air battery operation
Approach
The working principle of a photo-electrochemically rechargeable battery is similar to PEC water splitting except that sunlight is used to reduce Zn instead of releasing H2. The photoanode can produce a substantial photo-voltage under sunlight illumination that partly or completely offsets the charging voltage. In the ideal scenario, recharging might occur totally spontaneously. The photoelectrochemically rechargeable Zn-air batteries are a new concept as shown in Figure 2. The research project requires 1) a properly designed battery configuration that allows the integration of the semiconductor photoanode, and 2) development of semiconductor photoanodes with a large photocurrent, sufficiently negative onset potential and high stability in alkaline electrolytes.
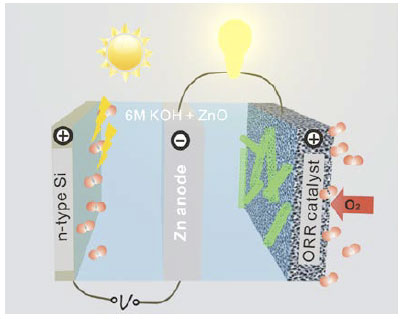
Figure 2: Schematic of a photoelectrochemically rechargeable Zn-air battery with an n-type silicon (Si) photoanode for water oxidation.
The research team will begin by synthesizing high-quality graphene oxide nanosheets or oxidized carbon nanotubes with different levels of oxidation and functionality. Then using a previous strategy, various metal-hydroxide or oxide nanoparticles will be grown on the electrode structures. These materials will be characterized using a wide range of spectroscopic and microscopic techniques, and assessed for electrochemical performance.
The optimal photoanode will be integrated into the battery in a three-electrode configuration to replace the electrocatalytic OER electrode on the left, as shown in Figure 1. The Zn electrode is connected to the electrocatalytic oxygen reduction electrode during discharge but switches to the semiconductor photoanode upon recharge under sunlight illumination. The team will carefully investigate the charging performance of the device by assessing the lowest charging voltage, maximum allowable charging current and stability, and comparing these key parameters with conventional Zn-air batteries. The ultimate goal of the proposed research is to increase the energy efficiency of rechargeable Zn-air batteries from less than 50% to 80-100%.