Molecular Genetics of the Inner Ear

Welcome to the Grillet Lab
RESEARCH
Genetics of Hearing and Vestibular Impairment
Hearing loss is a major health concern for our societies. In the United States of America, 17% of the adult population suffers from some degree of hearing loss. In addition, the elderly population frequently experiences debilitating hearing difficulties, and sometimes balancing issues.
Our goal is to identify the comprehensive list of genes required for hearing and head motion detection, and ultimately characterize the function of these genes at the molecular level.
This will improve our understanding of the inner ear physiology, but also will help the diagnosis of patients affected by hearing loss.
More about the Inner Ear
The inner ear contains the sensory cells that detect sound and head motion, the hair cells. In mammals, these cells are generated during the mid-gestation and will never be replaced during the entire life. The hair cells are in constant activity and their dysfunction is a major cause of deafness and peripheral vestibular disorders: they are both the core and the Achilles’ heel of the system.
More about the Genetics of Hearing loss
Hearing loss can result from exposure to excessive noise, chemicals and certain medications. However, susceptibility to deafness is generally dictated by genetic transmission. To this date, 136 human loci have been linked to hearing loss, but we know the corresponding affected genes for only 85 of them. These genes are very often required, directly or indirectly, for the proper hair cell function.
More about our Strategy
In order to identify these genes, we use mouse models either to precisely inactivate candidate genes (Reverse Genetics) or to generate randomly mutated animals and screen them for hearing or vestibular defects (Forward Genetics).
Using this later method, we identified the gene Loxhd1 as responsible for deafness in mice and humans (Grillet, AJHG, 2009), who are affected by a form of progressive hearing loss called DFNB77.

Differently from the sense of Vision, still little is known about Hearing and Balancing at their molecular level. This is due to the technical challenges associated with this organ: the paucity of the inner ear sensory cells, their inaccessibility and their fragility.
The inner ear is composed of two functional parts: the cochlea, which is the auditory organ, and the vestibule, organ responsible for head motion and balancing. In both parts, the sensory epithelia are composed of the sensory hair cells, called hair cells, always surrounded by supporting cells.
We want to characterize down to the molecular level the function of the cells that compose the inner ear, particularly the hair cells
More about Hair Cells
The hair cells have different functions:
1) to detect the mechanical stimuli induced by sound, and
2) to transmit this information to the central nervous system through their synapses.
More about Mechanotransduction
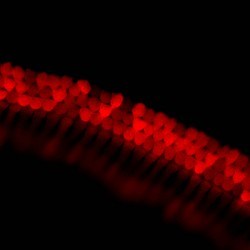
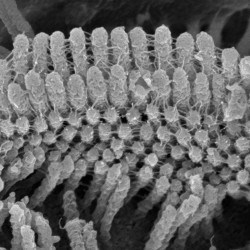
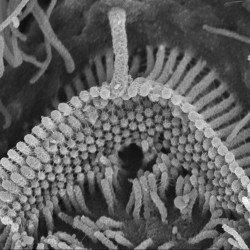
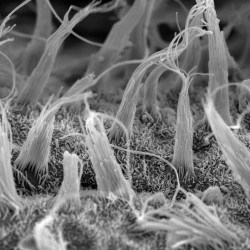
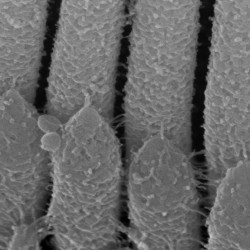
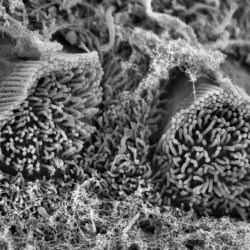
We are particularly interested in the organelle of mechanotransduction, the hair bundle. The hair bundle is located at the apical surface of the hair cells, consisting of a precise staircase arrangement of membrane protrusions filled with filamentous actin, bound to each other by extracellular linkages. Upon stimulation, force engages the bundle, and opening large conductance mechanically gated channels, generating the current that allows the sense of hearing and balancing. Cochlear hair bundles present different morphologies along the cochlea, differing from the elongated ones found in the vestibule.
We are interested in understanding the uniqueness of the hair bundles by identifying their key components and their functions.
More about our Strategy
In order to identify the key components of the stereocilia bundle we will be using different approaches such as mouse Genetics, Electronic Microscopy, Electrophysiology or Biophysics.
By using a mouse model, we have previously showed that the USH1C protein Harmonin is required for the proper sensitivity of the hair bundle to mechanical stimulation (Grillet et al., Neuron, 2009). More recently, we also have shown that the protein TMHS is associated with part of the mechanotransduction machinery and influences the mechano-channel properties (Xiong, Cell, 2012).



APPROACHES
ABOUT THE TEAM

Grillet Lab - April 2015
Grillet N, Schwander M, Hildebrand MS, Sczaniecka A, Kolatkar A, Velasco J, Webster JA, Kahrizi K, Najmabadi H, Kimberling WJ, Stephan D, Bahlo M, Wiltshire T, Tarantino LM, Kuhn P, Smith RJ, Müller U.
Mutations in LOXHD1, an evolutionarily conserved stereociliary protein, disrupt hair cell function in mice and cause progressive hearing loss in humans.
JOIN US
We are currently open to STUDENT ROTATION
and we also are offering a
POSTDOCTORAL
POSITION in
BIOINFORMATICS & GENOMICS
at the Stanford University
School of Medicine
THANK YOU FOR YOUR SUPPORT
CONTACT INFORMATION
Location &
Mailing Address:
Otolaryngology Department
Stanford School of Medicine
Stanford University
300 Pasteur Drive
Edwards Building
Lab: Room R213
Office: Room R113B
Stanford, CA 94305
Office: (650) 497-9393
Lab: (650) 498-5229
Fax: (650) 721-2163
Shipping Address:
Otolaryngology Department
Stanford School of Medicine
Stanford University
1291 Welch Road
(Edwards Building)
GRILLET LAB
Lab: Room R213
Stanford, CA 94305
Email: ngrillet@stanford.edu
Phone: (650) 497-9393
Fax: (650) 721-2163
DIRECTIONS
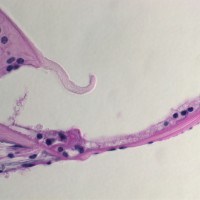
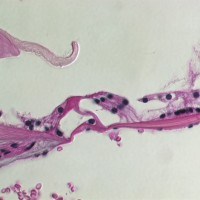
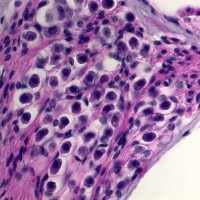
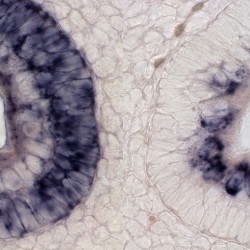
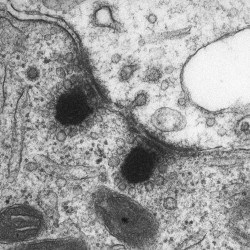
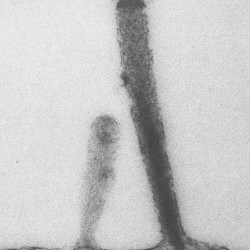
SCIENTIFIC PICTURES GALLERY
Sometimes pictures are more telling than words to describe scientific experiments.
We present here some images from our research on the inner ear.
These pictures are copyright protected; please contact the lab to obtain permission for use.
HISTOLOGY
MOLECULAR
BIOLOGY
MOUSE GENETICS
FLUORESCENCE MICROSCOPY
SCANNING EM
TRANSMISSION
E M
BIOINFORMATICS
COVERS