 |
|
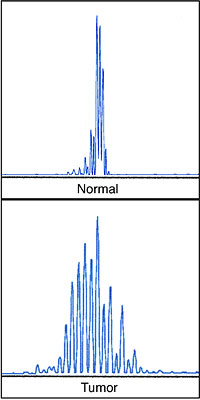
Microsatellite instability assay, performed in a patient with hereditary non-polyposis colorectal cancer (HNPCC). Microsatellite instability is present in the genetic marker shown and three others, confirming genomic instability in the tumor sample. |
|
Solid Tumors
Stanford Solid Tumor Actionable Mutation Panel (STAMP)
This assay detects potentially clinically actionable mutations, as well as mutations in hundreds of additional genes that are frequently mutated in solid tumors. STAMP is a targeted next generation sequencing method that uses target enrichment to capture genomic regions of interest. The targeted sequencing approach and integrated bioinformatics workflow is optimized for ultra-deep sequencing of formalin fixed tumor biopsy tissue specimens. The workflow includes acoustic shearing of isolated genomic DNA, followed by efficient preparation of sequencing libraries, and a target enrichment approach to capture genomic regions of interest for sequencing. The enrichment is done using custom designed libraries of capture oligonucleotides that target a specific set of genomic regions. This panel targets 198 genes, either in part or fully, with the genes selected on the basis of their known impact as actionable targets of existing and emerging anti-cancer therapies, their prognostic features, and/or their mutation recurrence frequency across patients with known cancer types. These genomic features are interrogated to achieve a minimum analytic detection-limit of at least 5%. Pooled libraries are sequenced on the Illumina® MiSeq system.
Genes entirely or partly covered:
Cancer Somatic Mutation Panel
CSMP is a next generation sequencing and SNaPshotTM system that covers 50 mutations common in a variety solid tumors and hematologic malignancies. The mutations detected with this assay are all potentially clinically actionable, typically involving signal pathway activating mutations which have targeted therapies available (some may require enrollment in clinical trials).
The SNaPshotTM methodology uses a single base extension step with a labeled ddNTP to detect 126 mutations in 12 genes commonly mutated in cancer.
A link to mutations covered by this assay:
http://stanfordlab.com/esoteric/cancer_somatic_mutation_list.htm |
Microsatellite Instability
Microsatellite instability (MSI) is detected in 90% of hereditary non-polyposis colon cancer (HNPCC) and 15% of the sporadic colorectal cancers. In many cases, DNA mismatch repair genes such as MSH2, MLH1 and MSH6 are defective. The laboratory performs a multiplex PCR amplification of 5 mononucleotide microsatellite markers; the size distribution of the products compared to normal tissue or peripheral blood leukocytes determines whether microsatellite instability is present. Immunohistochemical correlation is recommended in all cases as a supportive and confirmatory measure as suggested by the American Joint Committee on Cancer.
Hereditary Diffuse Gastric Cancer
CDH1 germline mutations are associated with both hereditary diffuse gastric cancer and lobular breast cancer. The inheritance pattern is autosomal dominant with incomplete penetrance (~80% for diffuse gastric cancer and ~40% for lobular breast cancer). Mutations are widely distributed throughout the gene, and associated with loss of function of the mutated E-cadherin allele. By the time gastric cancer becomes symptomatic, it is rarely curable (<20% 5 year survival). However, a high cure rate (>90% 5 year survival) is possible if the stomach is removed prior to tumor invasion through the gastric wall. Identification of individuals at high risk of developing diffuse gastric cancer affords the opportunity for elective prophylactic gastrectomy. Direct DNA sequencing that covers coding and splice site regions of the CDH1 gene is offered. Because mutations are distributed across all coding exons of the CDH1gene, an analysis of all exons is recommended. For families with a known mutation, sequencing of a single exon may be appropriate.
More information is available on the following website:
http://cancer.stanfordhospital.com/forPatients/services/geneticCounseling/HDGC
Other Solid Tumor Testing
The Stanford Molecular Pathology laboratory also offers testing for MGMT, KRAS, and BRAF.

