Research update: new therapeutic target
July 2, 2013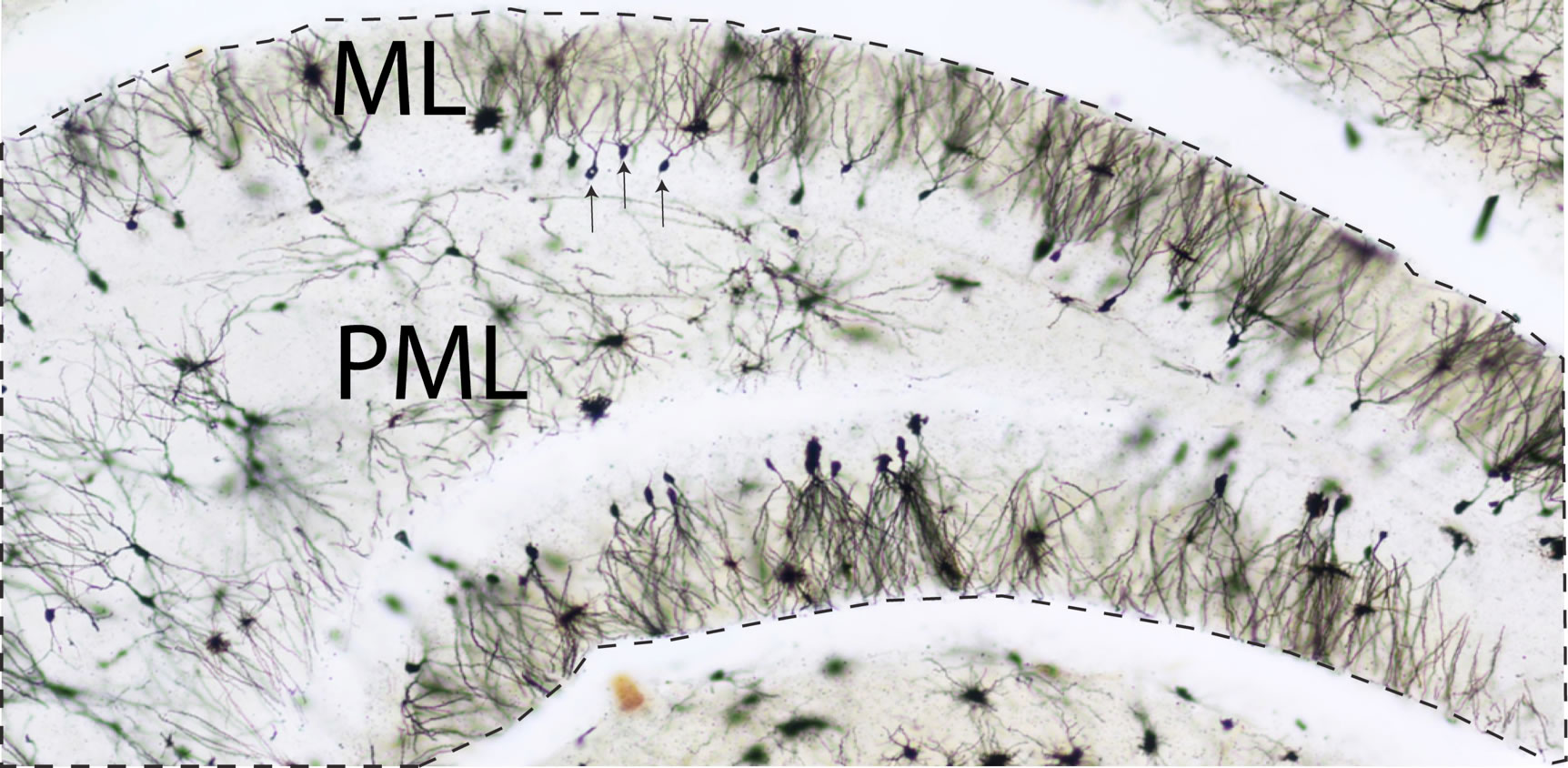
A new study from Stanford Down Syndrome Research Center affiliated member Dr. Ahmad Salehi describes a novel therapeutic strategy for intellectual disability in Down syndrome. Dr. Salehi and colleagues improved learning and memory in a mouse model of Down syndrome with injections of formoterol, a long-acting ß2 adrenergic receptor agonist. At much lower doses formoterol is approved to treat asthma and chronic obstructive pulmonary disease (COPD), but the formoterol dose used in this study cannot be safely used in humans without risking significant respiratory side-effects. Nonetheless, these results hold great promise for new classes of drugs targeting this receptor to address intellectual disability in Down syndrome.
In a study recently published in the July issue of Biological Psychiatry, the Salehi Laboratory (Dang et al., 2013) showed that the Ts65Dn mouse model of Down syndrome exhibited significant improvement in learning and memory after injections with formoterol, a long-acting ß2 adrenergic receptor agonist. According to the study, formoterol injections led to structural changes in the hippocampus and improved cognitive function in treated mice.
Formoterol is approved as a bronchodilator and in the treatment of asthma and chronic obstructive pulmonary disease (COPD), though at significantly lower doses than used in this study. In the lungs, formoterol works by relaxing smooth muscles in the airways. Generally, in addition to receptors in the brain, there are a large number of b adrenergic receptors in cardiovascular and respiratory systems. For these reason, systematic use of b adrenergic drugs would also cause significant peripheral side effects. The authors at the Stanford University Down Syndrome Research Center showed that this ß2 agonist can be safely given at a high dose when it is given in conjunction with a ß2 blocker (i.e. nadolol) that does not enter the brain, thereby blocking the effects of formoterol in the periphery.
Ts65Dn mice were generated to mimic Down syndrome in humans by carrying extra genes that are present on human chromosome 21. These mice have been widely used by scientists around the world to study neurobiological and molecular mechanisms responsible for cognitive disability in Down syndrome and develop new treatment strategies for intellectual disability in Down syndrome.
The hippocampus is a brain region critical for memory, spatial navigation, and attention. Damage to this region has been associated with various types of learning abnormalities. The dentate gyrus region of the hippocampus plays a significant role in contextual learning. This type of learning is generally achieved by integrating sensory and navigational information. For instance, finding the location of a coffee shop in a shopping mall is not only achieved by merely recalling the location of the place but also by learning about different sounds, colors, lights, and the presence of other shops nearby. The dentate gyrus supports contextual learning and spatial navigation by integrating sensory information from different sources in our surrounding environment.
Unfortunately, the dentate gyrus of the hippocampus is significantly affected in Down syndrome. Several studies have already shown that the dentate gyrus of the hippocampus exhibits significant shrinkage and abnormalities in people with Down syndrome. The Ts65Dn mouse model of Down syndrome also exhibits similar characteristic changes, such as the loss of dentate granule cells shortening of their dendrites.
In a previous study, published in Science Translational Medicine (Salehi et al., 2009), the authors reported that the locus coeruleus, an important group of neurons in the brainstem, undergoes significant shrinkage and loss of neurons during aging in Ts65Dn mice. The locus coeruleus is considered the sole source of norepinephrine, which activates adrenergic receptors (including the ß2 adrenergic receptor) and is an important neurotransmitter involved in learning and memory, for the hippocampus. Locus coeruleus neurons send extensive projections to the cortex and hippocampus, and modulate the function of the target regions by releasing norepinephrine. For this reason, any minor abnormalities in locus coeruleus neurons could lead to drastic reductions in norepinephrine levels in the hippocampus and significant functional abnormalities in this region.
Previously, the same group had shown that l-threo-3,4-dihydroxyphenylserine (L-DOPS), a norepinephrine precursor, could effectively restore contextual learning in the Ts65Dn mouse model (Salehi et al., 2009). However, L-DOPS has not been approved for clinical use by the FDA. For this reason, Dang and colleagues tested whether targeting b adrenergic receptors more directly would also be effective in restoring contextual learning and improving synaptic density (neuronal connections) in the hippocampus of Ts65Dn mice.
Norepinephrine acts in the brain by binding to specific a and b receptors. Among the three b-adrenergic receptors, ß1 and ß2 are most prominent in the hippocampus. These receptors are also found in the periphery outside the brain. Unlike ß2 receptors that are predominantly found in the respiratory system, ß1 adrenergic receptors express in the cardiovascular system. While activation of ß1 adrenergic receptors leads to increased blood pressure and heart rate, ß2 receptor activation leads to increased respiratory rate and improved respiratory function. A large number of people with Down syndrome are afflicted with various cardiovascular system abnormalities; therefore, it is probably prudent to avoid using drugs that target this system.
Formoterol has been used for the treatment of respiratory dysfunction including asthma in adults and children. However, a much higher dose was used in the current study. To limit the effects of formoterol in the body, it was only given after a ß blocker was used in these mice. This blocker (called nadolol) cannot cross the blood brain barrier and therefore only blocks ß receptors outside of the brain, thereby mitigating side effects of formoterol. As expected, the authors found no significant effects of formoterol on vital signs including respiratory rate, hearth rate, and oxygen saturation levels in the treated mice.
To test the effects of formoterol on structure and function of the hippocampus, the brains of young adult Ts65Dn mice and their controls (2N) were thoroughly analyzed. The authors found a significant reduction in the hyperactivity and an improvement in contextual learning after formoterol treatment in the Ts65Dn mice. In addition, the treatment led to restoration of the density of neuronal synapses (intra-neuronal connections) and a significant improvement in branching patterns of neuronal dendrites in the hippocampus in Ts65Dn mice.
The doses of ß2 adrenergic agonists used in this study are not safe for use in humans, and more research is needed before formoterol can be tested in people with DS. Of note, more than 70% of people with Down syndrome already suffer from sleep apnea, suggesting that respiratory abnormalities might also play a significant role in cognitive dysfunction in people with Down syndrome. For this reason, using drugs that also improve the respiratory function in people with Down syndrome may be very attractive in more than one way. Future drug development to identify compounds effective for normalizing both respiratory and cognitive abnormalities in Down syndrome could be transformative. Thanks to decades of research and development focused on ß adrenergic drugs by pharmaceutical companies, there is a great hope that new, more powerful, safer, and more effective ß2 adrenergic drugs with the ability to cross the blood brain barrier will be developed in the future.
The study was funded by the Down Syndrome Research and Treatment (DSRTF) and Research Down Syndrome.

