Medical linear accelerator celebrates 50 years of treating cancer

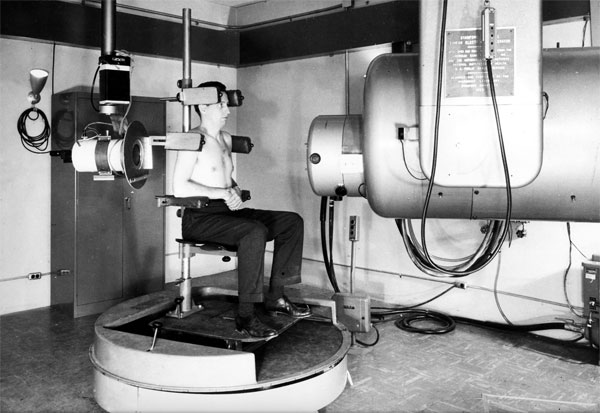
Patients get state-of-the-art cancer therapy from a modern linear accelerator in the Stanford Comprehensive Cancer Center. The accelerator, which was developed at Stanford, will celebrate its 50th anniversary at an April 19 symposium.
Lucky for one young cancer patient in 1956 that Henry Kaplan, MD, had already come to Stanford with an unusual goal: to turn a device used by the physicists on campus—the linear accelerator—into a tool for fighting cancer.
That patient, a 2-year-old boy suffering from a tumor in his eye, was the first to undergo X-ray treatment from a medical linear accelerator that Kaplan developed with campus physicists. The treatment saved the child's sight and he lived the rest of his life with his vision intact.
Fifty years and 40 million patients later, medical linear accelerators have become the backbone of radiation therapy for cancer worldwide. Roughly half of all cancer patients receive radiation therapy, primarily from the rays generated by a linear accelerator.
In recognition of the 50th anniversary of the medical linear accelerator, the Department of Radiation Oncology is presenting a celebratory symposium on April 19.
The device also has a number of other medical uses. Its radiation can quell the rejection of an organ transplant, suppress the immune systems of patients undergoing blood and marrow transplantation, and correct certain neurological and cardiovascular disorders.
The medical linear accelerator is a dramatic example of what can be achieved when researchers from different academic disciplines converge to solve a problem. This 50-year-old achievement was a precursor to the multidisciplinary approach now called translational medicine.
"I characterize the linear accelerator as one of the most important examples of translational research that has occurred at this institution and around the world," said Richard Hoppe, MD, the Henry S. Kaplan-Harry Lebeson Professor of Cancer Biology and chair of radiation oncology.
It all came about because Kaplan thought he could find a way to focus intense X-rays to destroy a tumor without harming surrounding healthy tissues—and he thought the physicists would be the ones to help him do so.
"In the 1950s, I began to hear cocktail party conversations about an interesting new atom smasher being developed on the campus by Bill Hanson and Edward Ginzton and their colleagues," Kaplan recalled in an 1983 interview, the year before his death. The interview appeared in The World of Stanford Radiology, a 2006 book by Otha Linton that chronicled the 100-year history of radiology at the School of Medicine.
An atom smasher, otherwise known as a particle accelerator, uses electromagnetic fields to propel charged particles to great energies. By slamming accelerated electrons into a target made of heavy metal, high-energy X-ray beams result. Kaplan thought this machine could be harnessed to deliver X-ray therapy that was superior to the unreliable, weak and unfocused radiation therapy approaches of his day.
"Ed Ginzton and I spent our first luncheon talking about the properties of the Stanford linear accelerator," continued Kaplan, who was chair of radiology from 1948 until 1972, in the book. "This sounded miraculous and I became convinced that this was to become the radiotherapy machine of the future."
Kaplan and Ginzton, PhD, professor of electrical engineering and of physics, developed the first medical linear accelerator in the Western Hemisphere, installed at Stanford-Lane Hospital in San Francisco. In January 1956 the machine was ready to be used on their first patient, a boy with retinoblastoma in his one remaining eye after surgeons had removed the tumor in the other eye. Destroying the tumor while sparing the eye would have been impossible with earlier, less-focused radiation sources.
"I will never forget the puzzled look on the face of the garage owner down on Fillmore Street when I asked him to borrow a heavy-duty automobile jack," recalled Kaplan in the book. "I explained that it was to carry a large block of lead with a pinhole in it, to enable us to position that pinhole day after day for six weeks directly opposite the tumor in the boy's eye while missing the lens and cornea."
In the years following this first patient treatment, Stanford radiologists continued to improve on the device's design, spurred by Kaplan's early ambitions of having his department's clinical science meld with the university's basic science departments of physics, engineering and biology.
Peter Fessenden, PhD, professor (teaching) of radiation oncology emeritus, arrived at Stanford as the head of the radiation physics section in 1972, just as the original accelerator was retired to the Smithsonian Institution after 16 years of patient treatment. Fessenden continued studies he had begun years earlier with the section's previous leader, exploring how to create linear accelerators that delivered their punch to tumor cells through two types of radiation. Working with Varian Medical Systems Inc. of Palo Alto, his group helped develop the first machine that combined both X-ray and electron treatment.
"We learned a heck of a lot from this machine, the dos and don'ts," said Fessenden, who retired in 1995. "Using a lot of that information, Varian went on to develop the precursor to all the modern machines you see now." Current machines have two or three energies of X-rays and up to seven energies of electrons, giving physicians versatility for crafting the most effective attack.
"One of the biggest challenges we have is aligning the radiation beam and the tumor," said Paul Keall, PhD, the current head of radiation physics. The heart beating, the lungs inflating and deflating with each breath, the blood flowing through the veins, subtle movements of the skeleton or muscles—all this motion adds up to a moving target for a beam of deadly radiation.
A linear accelerator was co-developed at Stanford that can continually track the position of tumors in real time during treatment. Tracking allows clinicians to deliver larger doses of radiation precisely to the tumor. Called the Cyberknife, the device was first used for patient therapy in 1994. Many of the current studies continue to refine how to account for the anatomical changes of a patient with time, from second-by-second to day-by-day, said Keall.
Stanford also has dozens of clinical trials in progress investigating new ways to use radiotherapy for cancer treatment and immunosuppression. The radiation oncology group has a long history of exploring other sources of radiation instead of X-rays and electrons—such as negative pi mesons, the subatomic particles that Fessenden studied. Now researchers are exploring the use of protons and carbon ions.
When you look at modern medical linear accelerators, said Hoppe, "they are light-years ahead of that unit built by the physicists at Stanford in the early 1950s." But what is in store for a cancer patient 50 years from now?
The next generation of radiotherapy will encompass the techniques of molecular imaging, which allows doctors to differentiate tumor from normal tissue.
"We are developing tools that allow us to deliver more radiation into the parts of the tumor that are most resistant to radiation," said Keall. "So there is quite a good fusion here of the molecular imaging and biology and the physics, which can be translated to better cancer treatment through the use of the linear accelerator."
Symposium highlights
In recognition of the contributions that the medical linear accelerator has made to cancer treatment, the Department of Radiation Oncology is hosting a celebration, "A culture of innovation: Celebrating pioneering efforts of the past and envisioning the future," on April 19. The invitation-only event will include a symposium, tours of the Stanford Comprehensive Cancer Center, a poster session and reception.
The symposium's speakers are:
History of the medical linear accelerator:
1952: Henry Kaplan and Edward Ginzton begin building a medical linear accelerator.
1956: The first medical linear accelerator in the Western Hemisphere is installed at Stanford Hospital in San Francisco.
1959: Stanford medical school and hospital move to the Palo Alto campus, bringing the medical linear accelerator.
1962: Kaplan and Saul Rosenberg begin trials using the linear accelerator with chemotherapy to treat Hodgkin's disease, an approach that dramatically improves patient survival.
1994: First use of the CyberKnife, invented at Stanford, which uses sophisticated computerized imaging to aim a narrow X-ray beam precisely.
1997: Stanford pioneers the use of intensity-modulated radiation therapy, which combines precise imaging with linear accelerators that deliver hundreds of thin beams of radiation from any angle.
2004: Implementation of four-dimensional radiotherapy, which accounts for the motion of breathing during imaging and radiation delivery.
2006: 50th anniversary of the Stanford medical linear accelerator.



Share This Story