Start Date: September 2012
PDF version
Investigators
Robert Waymouth and Christopher Chidsey, Department of Chemistry, Stanford University
Objective
The goal of this project is to develop efficient strategies for using renewable sources of electricity to produce energy-dense liquid fuels from carbon dioxide (CO2) and biomass. Researchers will develop new families of electrocatalysts for the electrohydrogenation of CO2 to carbon-neutral fuels.
Developing a broad understanding of the energetics and kinetics of efficient electro-hydrogenation is an important frontier in chemistry. If successful, this project will provide new scientific insights that will expand the range of technology options for reducing global greenhouse gas emissions by producing carbon-neutral fuels for the storage of renewable electricity, transportation and other sectors where carbon-based fuels dominate. Efficient electro-hydrogenation catalysts for CO2 have the potential for application at significant scale, particularly at sites where renewable but intermittent electricity from solar or wind energy is available.
Background
Carbon-based liquid fuels are currently the most efficient and versatile energy carriers for high- power, mobile energy needs. Developing new strategies to convert clean electricity to liquid fuels is a critical frontier in the transition to a renewable energy economy. One potentially game-changing technique is electrohydrogenation – the use of an electrocatalyst to drive the chemical reaction between a molecule and hydrogen.
The goal of this project is to develop technologies that use electricity from renewable sources to (1) convert CO2 into carbon-neutral fuels, such as methanol, or (2) transform biomass into long-chain molecules that can also be used as fuel.
CO2 is kinetically and thermodynamically inert, making reduction by chemical hydrogenation or electro-reduction very difficult. The products of CO2 partial reduction, formic acid and formates, are likewise resistant to reduction.1 CO2 electro-reduction studies date back to experiments done on zinc more than a century ago.2, 3 In general, large overpotentials are required that are thought to result from a reduced CO2–intermediate that is only partially stabilized by association with the metal surface.2
Approach
Insights gained over the last decade in chemistry will be used to discover new classes of energy-efficient electrohydrogenation catalysts. Research will focus on three areas– chemical hydrogenation of CO2, transfer hydrogenation chemistry and electrochemical reduction of CO2 – to develop new approaches to CO2 activation and electrochemical hydrogenation at low overpotential. Transfer hydrogenation catalysis (Figure 1) is a promising strategy for hydrocarbon reduction that occurs rapidly with high-energy efficiency (low thermodynamic driving force).
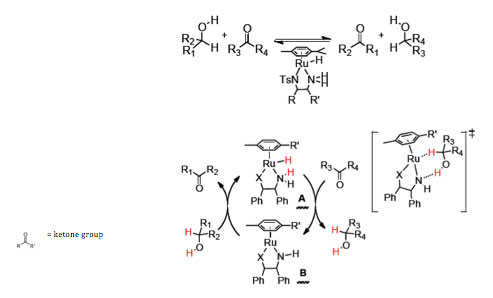
Figure 1: Schematic of the transfer hydrogenation of ketones with alcohols. Catalysts hydrogenate ketones with isopropanol or formic acid as the hydrogen source.4, 5
Ligand-assisted transfer hydrogenation mechanisms provide promising leads for the efficient and rapid rearrangement of two electrons and two protons from a metal complex to a C=O double bond. These strategies will be coupled with insights derived from proton-coupled electron transfer.
A cross-cutting study of metal catalysts that could be electro-reduced to metal hydrides capable of rapid and efficient hydrogenation of C=O bonds will be carried out. These metals will be incorporated into coordination complexes in which the metal hydrides can be readily formed electrochemically from protons and electrons. This technique will allow the metal hydride and a proton on a ligand of the complex to react with C=O bonds by low-energy pathways.
To upgrade biomass to fuels, researchers will target the electro-hydrogenation of the C=O bonds in CO2, carbonates, formates and C=O-containing compounds derived from carbohydrates.
References
[1] Balaraman, E., et al., “Efficient hydrogenation of organic carbonates, carbamates and formates indicates alternative routes to methanol based on CO2 and CO” Nat Chem 2011, 3, 609-614.
[2] Whipple, D. T.; Kenis, P. J. A. “Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction” J. Phys. Chem. Lett. 2010, 1, 3451-3458.
[3] Benson, E. E.; Kubiak, C. P.; Sathrum, A. J.; Smieja, J. M. “Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels” Chem. Soc. Rev. 2009, 38, 89-99.
[4] Ikariya, T.; Blacker, A. J. “Asymmetric transfer hydrogenation of ketones with bifunctional transition metal-based molecular” Acc. Chem. Res. 2007, 40, 1300-1308.
[5] Haack, K. J.; Hashiguchi, S.; Fujii, A.; Ikariya, T.; Noyori, R. “The catalyst precursor, catalyst, and intermediate in the Ru-II-promoted asymmetric hydrogen transfer between alcohols and ketones” Angew. Chem., Int. Ed. 1997, 36, 285-288.