Basic Science
- Tim Angelotti, MD, PhD
- Martin Angst, MD
- Edward Bertaccini, MD
- David Clark, MD, PhD
- David Drover, MD
- Rona Giffard, MD, PhD
- Eric Gross, MD, PhD
- Bruce MacIver, PhD
- Sean Mackey, MD, PhD
- Andrew Patterson, MD, PhD
- Ronald Pearl, MD, PhD
- Gary Peltz, MD, PhD
- Gregory Scherrer, PharmD, PhD
- James Trudell, PhD
- David Yeomans, PhD
- Obstetric Anesthesia Research Group
Timothy Angelotti, MD, PhD
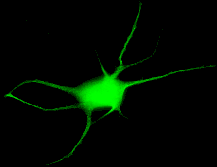
My research efforts are focused on investigating the pharmacological and physiological interface of the autonomic nervous system with effector organs. Utilizing molecular, cellular, and electrophysiological techniques, I am examining the function of a2 adrenergic receptors in sympathetic neurons cultured alone, or in the presence of other neurons, cardiac myocytes, or smooth muscle cells. Sympathetic neurons are isolated from various transgenic knock-out and knock-in mice, which have been designed to express altered adrenergic receptor subtypes (e.g. a2A/C, b1, b2). Using recombinant adenovirus constructs encoding additional wild-type, mutant, or chimeric adrenergic receptors, I am able to further modify the pharmacology and physiology of this system. Receptor binding, immunocytochemistry, single-cell analysis of neurotransmitter release, and standard molecular biology are the techniques that are employed to characterize this dynamic system. Development of this in vitro model of the sympathetic nervous system will allow us to better understand its role in sympathetically-mediated pain and in the stress response to surgery and critical illness.
E-mail Dr. Angelotti
Martin Angst, MD
Our laboratory's current transformative research efforts focus on studying immune health in the context of surgery and anesthesia. Our interest stems from previous work elucidating the modulation of inflammatory responses by anesthetic and analgesic drugs.
The aim of studying immunity in patients undergoing surgery is to identify immune phenotypes predictive of aversive postoperative outcomes including protracted recovery and infection. Our major working hypotheses are that 1) specific immune phenotypes will predict the risk for developing postoperative complications, 2) immune phenotyping will lead to the discovery of mechanisms aggravating or alleviating such risk, and 3) gained knowledge will allow devising immune-modulatory strategies mitigating such risk. Mass cytometry (CyTOF), proteomics, and functional ex-vivo immune assays are the major molecular tools for the systems-based numerical and functional exploration of the circulating immune system.

E-mail Dr. Angst
David Clark, MD, PhD
Current projects being pursued in the Clark lab can generally be placed in one of to categories. One group of projects involves the investigation of the roles of heme oxygenase in nociceptive mechanisms. To this point we have demonstrated a role for this enzyme in many rodent pain models including models of inflammatory, incisional and neuropathic pain. We are currently addressing issues related to specific spinal neurotransmitter systems which may be modulated by heme oxygenase, examining the role of heme oxygenase in modifying analgesic responses to opioids, and determining the extent and nature of the interactions between heme oxygenase and nitric oxide.
The second group of projects involves the mechanistic exploration of opioid-induced hyperalgesia. Experiments from our lab have to this point demonstrated thermal hyperalgesia and mechanical allodynia in mice and rats after the cessation of opioid administration. This hyperalgesia has been partially characterized pharmacologically. Ongoing studies seek to further elucidate the mechanism of this form of hyperalgesia as well as test methods for preventing or limiting its manifestation. We are currently using behavioral, immunohistochemical and biochemical methods.
E-mail Dr. Clark
David Drover, MD
My research interest is in clinical research on the pharmacokinetics and pharmacodynamics of drugs. Medications studied are those commonly used for anesthesia and analgesia. Additionally, other drugs are studied if they have unique characteristics that require intensive or specialized monitoring. Particular effort is used to obtain quality real-time data from intensive pharmacokinetic - pharmacodynamic studies to enable mathematical modeling of drug effect on the human body. Mathematical modeling of data is mainly performed with NONMEM. Where possible, research projects use the electroencephalogram to quantitate pharmacodynamic effect and develop mathematical models to relate pharmacokinetics to pharmacodynamic response. The main interest of my research projects is to develop novel ways to model and describe clinical pharmacology relationships.
E-mail Dr. Drover
Rona Giffard, MD, PhD
Dr. Rona Giffard's laboratory studies stroke. Stroke is a devastating problem that is the leading cause of long term neurological disability and the third leading cause of death worldwide. We try to identify ways to reduce ischemic brain injury, and to better understand the interactions between different brain cell types during injury and recovery. Astrocytes and their response to injury is an important focus in the lab. We have found that astrocyte impairment contributes to neuronal injury in global ischemia. Targeting protective strategies to astrocytes leads to markedly increased neuronal survival.
Another area of focus in the lab is the increase in neurogenesis following stroke, and the deleterious effects of inflammation on neurogenesis in this setting. We are studying ways to improve mitochondrial function to increase newborn neuron survival, and modulate inflammation.
MicroRNAs are small noncoding RNAs that reduce translation and thus inhibit gene expression. Recent work shows that microRNAs are regulated in response to stroke, and may play an important role in neuroprotection. We are studying microRNAs that regulate important survival proteins including members of the Bcl-2 family and heat shock proteins. Because each microRNA can target multiple messengerRNAs, this is a way to target several physiologically related genes at once.
Inflammation following stroke while having important necessary roles, can also contribute to worsening injury. We have investigated the importance of IL4 in stroke, and found that it protects male mice from stroke injury. Results in females however, differed. Biological sex differences are seen in differences in the age of stroke and outcome to stroke in patients. We are also investigating sex differences in response to stroke, with a focus on differences in inflammation.
Studies are performed in animal models and primary cultures from brain.
E-mail Dr. Giffard | giffardlab.stanford.edu
Eric Gross, MD, PhD
Our laboratory focuses on developing non-narcotic cardiac-safe pain therapeutics and other next generation therapeutics for anesthetic and analgesic care. Further, in order to provide overall better anesthetic care for our patients, we are also examining how common genetic polymorphisms in our patient population we care for may alter anesthetic and analgesic effects of the medications we administer and the post-operative course.
In order to optimize analgesics and limit side-effects, we are also interested in investigating the mechanism of how the nociceptive and cardioprotective signaling pathways are linked. This involves studying the role of nociceptors in cardiac protection and continued interest examining the mechanism of how opioids and volatile anesthetics protect tissue from ischemia-reperfusion injury.
E-mail Dr. Gross | med.stanford.edu/grosslabBruce MacIver, MSc, PhD
The long-term goal of our research is to provide physiological background information required for the rational design of safer and more effective anesthetics and analgesics.
Hippocampal Research
We investigate the cellular, synaptic and molecular mechanisms of action of central nervous system drugs; especially barbiturates, opiates, anesthetics and other CNS depressants. Electrophysiological recording techniques and selective pharmacological probes are used to investigate the sites and mechanisms of action for CNS depressants. Most of our studies focus on the CA 1 area in rat hippocampal brain slices. Neurons in this brain area are depressed by anesthetics through a combination of pre- and postsynaptic actions on glutamate and GABA mediated neurotransmission.
Theta Research

The effects of pharmacological agents on EEG waves generated by the neocortex are also being examined. EEG theta activity (4 to 12 Hz) is one of many rhythms, like alpha and delta (slow wave sleep) rhythms that are altered by anesthetics. Patch clamp and electrophysiological recording techniques are used to look at the effects of anesthetics on carbachol and bicuculline induced theta activity in neocortical brain slices. Anesthetic effects on brain slice micro-EEG activity are correlated to EEG effects seen in animals and humans during anesthesia. Effects on micro-EEG theta activity were shown to involve actions at GABA and glutamate synapses.
Theta activity can be recorded from specific regions (green dots) of cortex in rat brain slices. Comparison of micro-EEG signals and intracellular recordings (whole cell) reveal that the low frequency theta waves (~ 8 Hz) were generated by synchronous synaptic potentials and discharge activity of cortical neurons. The discharge of each cortical neuron appears to contribute ~ 1.0 ÁV to the micro-EEG signal, so theta activity requires synchronous activity in ~ 100 neurons in each cortical location. Theta activity is known to be important for spacial mapping and may provide a 'binding' mechanism that contributes to the formation of memory in general. When selective populations of neurons are synchronously active they can interact in a Hebbian manner to change the strength of synaptic inputs that are timed at the theta frequency. Theta activity is also known to be particularly sensitive to anesthetic agents at concentrations which block memory formation. Preliminary studies in our laboratory indicate that brain slice theta activity is also depressed by anesthetics and that this depression occurs with a profile similar to in vivo responses.
E-mail Dr. MacIver | http://www.stanford.edu/group/maciverlab/
Andrew Patterson, MD, PhD
My research focuses on cardiovascular physiology. I am interested in the roles of adrenergic receptors in both normal and diseased hearts. I am also interested in developing gene therapy techniques that might be used to treat heart failure. In addition, my laboratory group has collaborated on several projects designed to elucidate the mechanisms of vasoregulation. The techniques employed in my laboratory range from microsurgery to gene therapy to ECG telemetry to exercise treadmill testing using mice.
E-mail Dr. Patterson
Ronald Pearl, MD, PhD
My research examines mechanisms and therapy of experimental pulmonary hypertension. We use the combination of pneumonectomy and monocrotaline administration to produce proliferative pulmonary hypertension in rats. We are currently examining the changes at a transcriptional and cellular level which result in pulmonary hypertension and the ability of vasodilator, immunosuppresive, and antiproliferative therapies to prevent and/or reverse the pulmonary hypertension. Ongoing research will develop a model of pulmonary hypertension in genetically altered mice and the ability of gene therapy to cure pulmonary hypertension.
E-mail Dr. Pearl
Gregory Scherrer, PharmD, PhD
Our laboratory investigates the cellular and molecular mechanisms of pain and its control by opioids. We want to identify the neural circuits that underlie pain perception and to resolve the molecular mechanisms by which opioids regulate activity in these circuits to generate analgesia. Our ultimate goal is to uncover new therapeutic strategies to treat chronic pain and opioid-induced side effects. To this end we combine a variety of experimental approaches including molecular and cellular biology, neuroanatomy, electrophysiology, optogenetics and behavior.
E-mail Dr. Scherrer | Lab site »
James Trudell, PhD
My research interests include usage of computational chemistry to develop molecular theories of anesthesia. I collaborate with three groups that use molecular biology to make site-directed mutations in ligand-gated ion channels. Molecular modeling of these channels is used to visualize the effect of mutations and to predict new mutations that will further refine their structure. I also use quantum mechanics calculations to determine the kinds of interactions that are likely to provide binding energy for anesthetic molecules at their sites of action.
ANESTHETIC INTERACTION WITH MEMBRANE PROTEINSJIM TRUDELL, PH.D., EDWARD J. BERTACCINI, MD
At the turn of the last century the Meyer-Overton relationship was proposed that relates anesthetic potency to the fat solubility of the anesthetic. This relationship fueled a large academic effort to find the mechanism of anesthetic action. Not surprisingly much attention was focused on determining whether the site of anesthetic action was the lipid layer of the plasma membrane where anesthetics would in some way perturb the lipid bilayer structure. While the results did explain some of the characteristics of anesthetic action there were also many deficiencies. The attention then shifted to the lipid-protein interface where it was thought that anesthetics may disturb the lipid microenvironment around the protein and thereby change the function of the protein. Recently most research has centered on anesthetics directly interacting with membrane proteins and thereby causing a change in their function. Unfortunately anesthetics do not have a high enough affinity for proteins to study the interaction using classical biochemical means. So if the anesthetics were going to play tough and be elusive, the researchers have to be equally inventive. Bring in the big guns: computational chemistry to develop molecular theories of anesthesia.
Drs. Jim Trudell and Ed Bertaccini have been using the methods of bioinformatics, structural biology and computational chemistry to build 3 dimensional models of the various multi-subunit ligand-gated ion channels through which anesthetics are thought to mediate their effects. Much of their work has been focused on the glycine and GABAA receptors found in the brain and spinal cord because they have been shown to play a role in anesthetic action. By doing this extensive modeling study they have developed the first three dimensional visualization of an anesthetic binding site within a clinically relevant protein. Using computational chemistry techniques, they have mapped out this binding site so as to determine the chemical requirements for anesthetic binding. They collaborate with three groups that use molecular biology to make site-directed mutations in these ion channels. The original models are then tweaked to include the modification of the protein sequence and the binding pocket is reexamined. Molecular modeling of these channels is used to visualize the effects of mutations, to predict new mutations to be made and test their properties. This new information will be used to further refine the 3-D structure of the protein.
The results of this work are best seen by looking at the computer images of the receptor model with and without anesthetics. They found a binding pocket in the midst of the protein where there is room for the anesthetic to sit. It seems the anesthetic is held there by weak forces exerted by lipophilic and hydrophilic amino acid residues. If the amino acids within that pocket were modified to make them bigger they could either mimic or block the action of an anesthetic. Even more intriguing-, if the subunits of the receptor are allowed to move in the model, the effect of the anesthetic on the ion channel pore may become apparent.
So will we be better off knowing how inhalational anesthetics work? The answer to that may be yes as knowing how anesthetics work may make it possible to design better anesthetics- ones with more selective and specific actions and ones that might also be more readily reversible.E-mail Dr. Trudell
David Yeomans, PhD
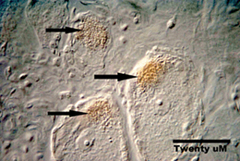
We are leading the way with two potentially revolutionary approaches to the treatment of chronic pain, namely transplantation and gene therapy. In the first approach, cells taken from the adrenal gland are transplanted on top of the spinal cord through a spinal needle. These cells make and secrete numerous natural analgesic substances, acting like a pump to produce a constant inhibition of pain. Unlike a pump however, these cells are alive, meaning that they keep making and secreting the analgesic chemicals for months.
The primary problems in gene therapy have been the targeting of the right cells, and the duration of the desired effect. We have used a highly modified herpes simplex virus (the kind that causes cold sores) to carry analgesic genes into the pain sensing cells. Because herpes viruses stay in these cells for the life of the host naturally, we should obtain very long lasting analgesic effects using these treatments. Thus, we are making use of the natural proclivity of herpes for entering and staying in the very cells we are interested in. In this way, we target pain treatment to painful areas, and only painful areas. These new approaches to therapy could revolutionize the treatment of chronic pain.
E-mail Dr. Yeomans
Obstetric Anesthesia Research Group
The obstetric anesthesia research group addresses a wide variety of questions related to the field. Two areas are currently our major focus:
- The use of spinal opioids for epidural and spinal anesthesia and analgesia.
- Spinal anesthesia for cesarean delivery.
In addition, we have done research on the economics of obstetric anesthesia, patient perception of risk, experimental pain, and the use of nitroglycerin for preterm labor and external cephalic version.
We have a large number of faculty involved in research and generally have 1 or 2 post-doctoral fellows conducting clinical studies. We have successfully helped undergraduate students, medical students, and residents complete research projects. Our fellows come from our own residency program as well as other academic institutions, and we have provided research opportunities for several foreign scholars. Our past fellows have gone on to academic careers or been very successful in private practice.
One of the strengths of our group is our ability to collaborate with a variety of people in the institution. We have conducted projects in the past with Drs. Alex Macario (economics), Yasser El Sayed (Fetal-Maternal Medicine), David Drover (pharmacokinetics and statistics), and Martin Angst (experiment pain). In the future we hope to collaborate with Dr. David Yeoman's experimental pain laboratory. Regular interaction among the members of the group is the key to the success of our active group. This is facilitated by monthly research meetings and journal clubs. We have also developed a formalized mentoring program. Dr. Riley, the Section Chief directs the research program and, along with Dr. Sheila Cohen, (previous section chief and an experienced clinical researcher) meets regularly with the junior members of the team to discusses research and other issues.

