Start Date: December 2008
PDF version
Investigators
John Ralph, Xuejun Pan, and Sara Patterson, University of Wisconsin, Madison
Objective
To delineate a set of approaches for successfully altering lignin structure, in a way that allows plant cell wall breakdown to produce biofuels in more energy-efficient manner, by providing alternative plant-compatible monomers to the lignification process.
Background
Over the past decade it has become apparent that the metabolic malleability of lignification, the process of polymerization of phenolic monomers to produce lignin polymers, provides enormous potential for engineering the resistant polymer to be more amenable to processing. Massive compositional changes can be realized by perturbing single genes in the monolignol pathway, particularly the hydroxylases. More strikingly, monomer substitution in the process of lignification, particularly in cases where a plant’s ability to biosynthesize the usual complement of monolignols is compromised, has been observed. These substitutions include products of incomplete monolignol biosynthesis such as 5-hydroxyconiferyl alcohol, ferulic acid, and coniferaldehyde and sinapaldehyde. This suggests that lignin composition can be altered leading to plants with characteristics for improved processing to biofuels.
Approach
To delineate the best monolignol substitutes, promising plant-compatible precursors (monomers) will be synthesized and tested for their compatibility with lignification in biomimetic systems (Figure 1). Improvements in biomass processing from the incorporation of these new monolignols will be elucidated in biomimetic cell wall systems.

Figure 1: Biomimetically lignified cell walls – preparation of suspension-cultured maize cell walls. In a) and b) cell walls are stained with permanganate and viewed by Scanning Electron Microscopy. In b) lignification of the cell wall is observed in the presence of exogenously applied monomers, (as indicated by the darker staining lower band).
Plant materials will be evaluated for processing characteristics (Figure 2) and structurally characterized using NMR techniques (Figure 3), and improvements to monomer selection will be made iteratively. The range of novel monomers to be tested includes those in the following five classes:
- Difunctional monomers or monomer conjugates linked via cleavable ester or amide bonds.
- Monomers that produce novel cleavable functionality in the polymer.
- Hydrophilic monomers.
- Monomers that minimize lignin-polysaccharide cross-linking.
- Monomers that produce simpler lignins.
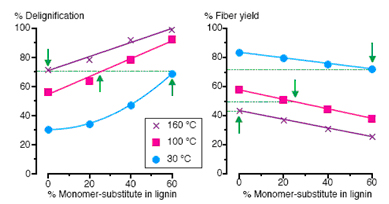
Figure 2: Delignification and fiber yields of synthetically lignified walls hydrolyzed with NaOH at different temperatures.
Those that are most promising will be revealed to other GCEP researchers so that the process of understanding the pathways that produce the monomers and obtaining the required genes can proceed most expediently.
 |
 Figure 3: Example of the use of Nuclear Magnetic Resonance (NMR) for lignin structural studies. Alfalfa plants deficient in the O-methyltransferase enzyme COMT, substitute 5-hydroxyconiferyl alcohol for sinapyl alcohol in lignin polymerization; a) Partial 2D 13C–1H correlative HMQC NMR spectra of lignins from i) the wild-type and ii) the COMT-deficient alfalfa. The major structural units (A-D, X1, and novel H) and color-coded chemical structures are shown. Dashed ovals in i) delineate the areas in which benzodioxane units H would correlate (if they were present). |