Stem cells are among the few cells in the body with the ability to generate new cell types, but because of this distinction, they are caught in a precarious situation. Stem cells walk a tightrope between the two most powerful cellular forces in the body: they are able to both self-renew, producing more stem cells, and differentiate, generating specialized cells with specific functions, such as blood or bone cells. If the balance tips in favor of self-renewal, stem cells can over-accumulate, in some cases leading to cancer. If the balance tips toward differentiation, the pool of stem cells can disappear, leading to the deterioration of organs.
Nowhere is that tightrope walk more vital or more perilous than in germline stem cells—the stem cells that ultimately produce sperm and eggs, or gametes. While all stem cells self-renew and produce specialized cells, germline stem cells (GSCs) are the only ones that make gametes, which are responsible for transmitting genetic information from one generation to the next. “They’re the only cells that you don’t actually need for your own survival, but you do need them for the survival of your species,” says Ruth Lehmann, an HHMI investigator at the Skirball Institute of Biomolecular Medicine at New York University.

In this way, GSCs are the very foundation of a species and must walk that tightrope carefully. “In a germline stem cell, just being precise isn’t enough,” says Yukiko Yamashita, an HHMI investigator at the University of Michigan. “To make sure your daughter can make a daughter who can make a daughter, it has to be perfect.”
To achieve that perfection, GSCs have evolved sophisticated strategies for their preservation and function. By studying those strategies, researchers have accomplished many firsts in stem cell biology, including identifying the first stem cell niches, discovering novel mechanisms of cell division, and pinpointing key signals that regulate stem cell actions. And many of the molecular pathways governing the precise balance between self-renewal and differentiation have been illustrated in vivid detail, making GSCs arguably the best understood stem cells in biology.
Walking the Line
Germline stem cells are a walking contradiction: they are ultimately able to generate every cell in the body but must produce only one type of cell—sperm or egg. “The germ cells are very primitive embryonic cells, and it’s really important for them to avoid somatic differentiation. They shouldn’t become muscle. They shouldn’t become nerve,” says Lehmann, who studies the very earliest stages of GSC formation in fruit flies.
From the very beginning, the precursors to GSCs, known as primordial germ cells (PGCs), are protected from becoming somatic cells by forming at the edge of an embryo, away from signals that might induce the wrong fate. This occurs by one of two mechanisms. In mice and other mammals, signals passed between extra-embryonic tissues and the embryo coax a specific set of cells at the periphery to become the germ cells. In flies, worms, frogs, and fish, on the other hand, a special protein-rich zone in the cytoplasm of the egg, called the germ plasm, determines which cells become PGCs. Germ plasm is a thick soup of proteins and RNA, with a much higher concentration of molecules than in the rest of the cytoplasm.
| Here, Ruth Lehmann discusses the strategies used by an embryo to specify which cells will become somatic cells and which will become germ cells. |
In 1992, at the Massachusetts Institute of Technology (MIT), Lehmann identified a key ingredient in that soup—a maternal effect gene she named oskar, which deposits a critical protein into the germ plasm. In Drosophila—those small, hairy fruit flies occupying laboratory vials since 1901 and a favorite for studying development—if oskar is inactive, no germ plasm forms, and no germ cells develop.
But when oskar is active, PGCs do form. From there, they adopt a clever defensive maneuver to avoid rapidly dividing and specializing like the rest of the cells in the embryo. The PGCs take a break; they pause.
This happens through extreme transcriptional repression, wherein DNA is prevented from being transcribed into RNA and is held temporarily silent, as if in a trance. Various molecular mechanisms control this repression, depending on the species, but the outcome is the same: little to no transcription and therefore no expression of the genome.
| Watch a brief video that explains the process of transcription. |
One of those mechanisms, recently discovered by HHMI Investigator Brad Cairns at the University of Utah, is the modification of the genome by chemical tags that bind and silence developmental genes, thus preventing differentiation. In a project that took more than four and a half years, Cairns’ team looked for and identified numerous modifications of DNA and histones—the proteins that package DNA—in multiple stages of male germ cell development in mice. They also validated their findings in human sperm.
Over time, the project became bigger and bigger. “We were astounded to find so many chemical marks and so many changes at different developmental stages,” says Cairns. “We made a major effort for several years, as each new result helped explain the logic of the regulation process.” As they reported in August 2014 in Cell Stem Cell, the group found a slew of tags—half “activating” tags and half “silencing”—stuck to key developmental genes. The activating tags help ensure that the genes can be turned on after fertilization. Until that time, the genes are held in check by the silencing tags.
|
|
| Learn more about the transcription factors, activators, and repressors that regulate transcription. |
With the DNA of genes promoting development in hibernation, some other system must regulate the activity of PGCs, which includes moving to the gonad and beginning to divide and produce daughter cells. It turns out that a hallmark of early germ cells is small, sticky balls of RNA and proteins that move around the cytoplasm and regulate RNA, leaving the DNA alone. Researchers believe that the DNA inside GSCs is so precious—the instructions for the survival of the species—that the DNA is kept in a pristine state in the nucleus, while the RNA is highly regulated by these sticky RNA-protein clumps in the cytoplasm. “Having less of an emphasis on transcription and more on RNA regulation may have something to do with preventing damage to the DNA,” says Lehmann. “We don’t really know, but it is an interesting phenomenon.”

In Drosophila, Pumilio and Nanos—proteins Lehmann discovered as a graduate student—are two of the proteins that compose these RNA-protein clumps, and they can be found in germ cells from worms to humans. In the worm Caenorhabditis elegans, Judith Kimble, an HHMI investigator at the University of Wisconsin-Madison, identified a Pumilio-like protein called FBF, required for GSC maintenance, which represses more than a thousand RNAs in germline stem cells, many of which activate developmental pathways.
A Niche Role
Kimble’s work on GSCs started much earlier. In 1981, as a postdoc at the MRC Laboratory of Molecular Biology in Cambridge, England, she used a laser to destroy cells in the tip of the developing gonad of C. elegans. By doing so, she identified a single cell—dubbed the distal tip cell—that was absolutely required for maintaining the worm’s GSCs. This special regulatory cell was not a germ cell but a somatic cell, and when Kimble moved it around the gonad, the GSCs moved with it. She was the first to show that a nearby somatic cell is essential for GSC maintenance—suggestive of a stem cell niche in C. elegans.
Subsequently, Kimble demonstrated that the distal tip cell niche maintains stem cells by producing a molecule that binds to a Notch receptor protein on the surface of GSCs. It was the first time that Notch signaling was shown to control stem cells. Researchers would go on to discover that Notch is a powerful regulator of many types of stem cells and an important driver of cancer.

Kimble and her team have since focused on unraveling the elaborate network downstream of Notch signaling, a series of about 100 molecules that together control the balance between self-renewal and differentiation. By identifying and manipulating those molecules, her team has been able to expand and contract the pool of GSCs in C. elegans.
Kimble has no plans to stop until she’s identified all the major links in the stem cell Notch signaling pathway. “I want the whole network that regulates this decision between self-renewal and differentiation,” she says. And she’s well on her way: just last spring, in the March 11, 2014, issue of Proceedings of the National Academy of Sciences, Kimble reported two new proteins essential for GSC maintenance that are direct targets of Notch signaling in GSCs—a long sought missing link between Notch regulation and the downstream regulatory network.
By the 1990s, most researchers believed in the existence of a stem cell niche—a specialized location inside a tissue where stem cells reside—but had never directly observed individual stem cells inside a niche. At the time, it was close to impossible to reliably isolate a single human—or mouse—stem cell, much less to identify where in the body the cell originated.
However, in Drosophila, the problem was tractable. “The small size and simplicity of their tissues make it easy to look at what individual cells are doing,” says Allan Spradling, an HHMI investigator at the Carnegie Institution of Washington. “Nowhere in all of biology, that we know of, is this more true than with the stem cells of the Drosophila ovary.”
Each fruit fly ovary contains 12 to 16 flexible tubes filled with chains of maturing egg cells. To locate the stem cells in the ovary, one simply follows a single tube back to its start, to the very tip where all the other cells originate. There reside two plump, round germline stem cells, the cells that produce eggs. Just how many animal species have female GSCs is still uncertain, though evidence suggests that female mammals, including humans, are born with all the eggs they are ever going to need.

Classical histologists identified Drosophila germline stem cells simply by looking under a microscope, but Spradling argued that the identity of such cells could be known with certainty only by tracing their cell lineages—that is, by identifying all the progeny of a single cell. In 1995, he verified the conclusions of the classical histologists about Drosophila ovarian GSCs by doing so, but in many other cases, such analysis showed that early guesses about other types of stem cells were not correct.
In 2000, he and postdoc Ting Xie also used lineage tracing to identify a germline stem cell niche in Drosophila. They marked ovarian GSCs with a gene encoding a fluorescent protein, making the cells glow under controlled conditions, and then manipulated them. When the researchers removed one of the two cells, the remaining cell replicated, and its offspring took the place of the absent stem cell. Spradling and Xie realized that the location, not some magical aspect of the cell itself, was key to the existence of a stem cell. “Our work showed a stem cell is really not a special cell; it is just an average, undifferentiated germ cell,” says Spradling. “The location made all the difference.”
Spradling then turned to the genes that might be involved in self-renewal and differentiation in the Drosophila ovary. He found one sterile mutation that caused the tip of each tube in the ovary to fill up with stem cells, like a bag of marbles. Naming the gene bag of marbles, or bam, Spradling’s team concluded that the gene is required for differentiation. Next, Spradling’s lab, in collaboration with the University of Texas Southwestern Medical Center lab of Dennis McKearin, now a senior scientific officer at HHMI, identified a signaling pathway in the niche that enables GSCs to silence bam and retain their stem cell identity. But when GSCs divide and daughter cells move outside the niche, they no longer receive the protein signal, so bam is active and the cells differentiate.
Orientation Matters
Around the time Spradling was identifying the signals regulating the female Drosophila niche, Margaret “Minx” Fuller, at Stanford University, was simultaneously studying GSCs in the Drosophila testis. Unlike the fruit fly ovary, the testis niche contains six to 12 germline stem cells clustered around a group of somatic support cells, called the hub, like petals on a flower. Yukiko Yamashita, then a postdoc in Fuller’s lab, decided to take a quick look at mitosis—the process of cellular division—in GSCs. Yamashita and Fuller hoped that by studying how GSCs divide, they might gain insight into how one of the offspring remains a stem cell while the other differentiates.

“We both planned just to have a tiny paper out of that project,” says Yamashita with a laugh. “That’s not what happened.” Yamashita found that the centrosomes, small, anchor-like organelles where the structural filaments needed for cell division originate, were not behaving as expected. Most biology textbooks show centrosomes separating after a cell commits to mitosis. Yet in the male fly GSCs, centrosomes were splitting apart from each other and moving around the cell before the initiation of mitosis. Because of this early movement, the centrosomes had marked where the mitotic spindle should be oriented—perpendicular to the stem cell niche, so that one cell stays in the niche and the other is pushed out—before mitosis even began.
“That [orientation] is probably responsible for the continued balance maintaining the stem cell population and continuing to produce [sperm],” says Fuller. “It is really critical for the stem cell program.” In retrospect, says Yamashita, it makes sense that germline stem cells would take this extra, early precaution to prepare for reliable stem cell division. Their “tiny paper” was published in 2003 in Science and has since been cited over 415 times.
Yamashita has continued to explore how one daughter cell stays in the niche to be a GSC while the other moves away, to commit to becoming a specialized sperm cell. “What kind of precise mechanism can make sure that only one cell of two daughter cells, next to each other, cheek to cheek, gets a signal to make it a stem cell?” Yamashita asks. “It’s like you have twin toddlers and try to give a snack to only one of them!”
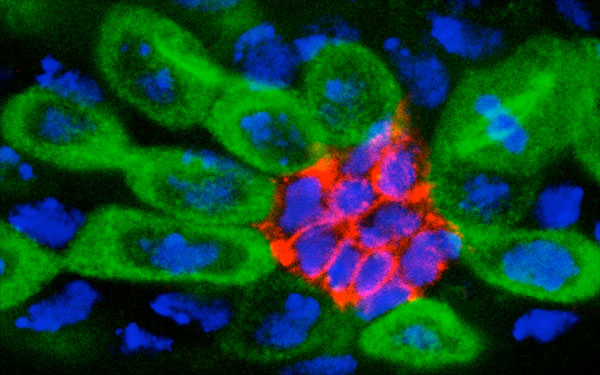
Recently, one of Yamashita’s postdocs came to her with a microscope slide and asked her to look closely. There, in Drosophila GSCs in the testis, she observed a slim whisker of microtubules, a nano-sized bridge poking out of the stem cell to touch the nearby stem cell niche. Yamashita was so surprised she went back to tissue images collected some 10 years earlier that were stored on her computer. Lo and behold, there was the same whisker. “It was there; I just hadn’t noticed it,” she says. “We missed that structure for such a long time.” The team investigated and discovered that a critical signal is sent from the niche cells to the GSC on the surface of that whisker—a precise mechanism for signaling to the stem cell but not the daughter cell.
Model Behavior
Once a daughter cell does leave the niche, it begins the process of becoming a viable gamete, which starts with meiosis—the process of cell division that leads to gametes, each with only half the genetic information needed to form an individual.
At the Whitehead Institute and MIT, David Page and colleagues have deciphered the signals that initiate meiosis in both male and female vertebrates, including the powerful Stra8 (Stimulated by retinoic acid gene 8), a gene expressed in the fetal ovary and juvenile testis (mammals continue producing sperm into adulthood, though not eggs), right at the time meiosis is initiated. “A defining feature of germ cells is they all know how to do meiosis,” says Page, “so I wanted to know how this super-conserved program is initiated.”

Page and his team have shown that retinoic acid, a fleeting and hard-to-track chemical believed to be produced by nearby support cells in the gonad, activates Stra8 in both ovaries and testes. In August 2014, Page’s team reported in PLoS Genetics that retinoic acid activates a second gene, Rec8, which encodes a protein also critical for meiosis and is conserved all the way from yeast to humans.
“There is so much now known from the fly and worm male and female germlines about [numerous] things happening in stem cells,” adds Fuller. “These have provided paradigms for people working in other adult stem cells—models for what kinds of things to look for.”















