CADDIS Volume 2: Sources, Stressors & Responses
pH
Related Links
On this page
Other sources/stressors/responses
Databases
Examples
Authors: G.W. Suter II, S. Cormier,
K. Schofield, J. Gilliam, C. Barbour

Courtesy of USGS Biology, Contaminant Biology Program.
pH is an expression of hydrogen ion concentration in water. Specifically, pH is the negative logarithm of hydrogen ion (H+) concentration (mol/L) in an aqueous solution:
pH = -log10(H+)
The term is used to indicate the degree of basicity or acidity of a solution ranked on a scale of 0 to 14, with pH 7 being neutral. As the concentration of H+ ions in solution increases, acidity increases and pH gets lower, below 7. When pH is above 7, the solution is basic. Because pH is a logarithmic function, one unit change in pH (e.g., from 7 to 6) indicates a 10x change in hydrogen ion concentration in that solution. However, what is actually measured is hydrogen ion activity, not concentration.
Note that although basic solutions are alkaline, “basicity” and “alkalinity” are not exactly the same thing. Basicity refers to the ratio of hydrogen and hydroxyl (OH-) ions in solution, and is directly related to pH. Alkalinity is related to the acid-neutralizing capacity (ANC) of a solution. In aquatic ecosystems, biological processes (e.g. decomposition) that increase the amount of dissolved carbon dioxide or dissolved organic carbon (DOC) decrease pH but have no effect on ANC.
pH affects most chemical and biological processes in water, and it is one of the most important environmental factors limiting the distribution of species in aquatic habitats. Different species flourish within different ranges of pH, with the optima for most aquatic organisms falling between pH 6.5-8. U.S. EPA water quality criteria for pH in freshwater suggest a range of 6.5 to 9. Fluctuating pH or sustained pH outside this range reduces biological diversity in streams because it physiologically stresses many species and can result in decreased reproduction, decreased growth, disease, or death.
Even small changes in pH can shift community composition in streams because pH alters the chemical state of many pollutants, such as copper and ammonia, changing their solubility, transport, or bioavailability. This can increase exposure to and toxicity of metals and nutrients to aquatic plants and animals.
Low pH
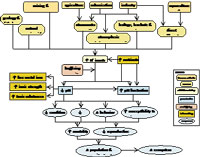
For a waterbody to be considered to have low pH, the pH generally needs to be below 6 or 6.5, depending on the system. Low pH is addressed in this module as a proximate cause of effects on aquatic biota. It should be listed as a candidate cause when potential human sources and activities, site observations, or observed biological effects support portions of the source-to-impairment pathways in the conceptual diagram for low pH (Figure 2). This diagram and some of the other information also may be useful in Step 3: Evaluate Data from the Case.
Checklist of sources, site evidence and biological effects
The checklist below will help you identify key data and information useful for determining whether to include low pH among your candidate causes. The list is intended to guide you in collecting evidence to support, weaken, or eliminate low pH as a candidate cause. For more information on specific sources and activities, site evidence, and biological effects listed in the checklist, click on checklist headings or go to the When to List tab of this module.
Consider listing low pH as a candidate cause when the following sources and activities, site evidence, and biological effects are present:
Sources and Activities
- Mine wastes
- Historic mine sites
- Acid-generating rocks/soils
- Power plants and other sources of acidic gases
- Coal pile runoff
- Industrial effluents
- Landfill leachate
- Confined animal feeding operations, dairy runoff
- Instream oxidation or reduction processes
- Recent draining of naturally inundated wetlands or floodplains
Site Evidence
- Low site pH data
- Acidic precipitation
- Geology or soil type (e.g., serpentine geology, soils)
- Mine drainage/overflows (current or historic)
- “Yellow-boy” (iron precipitates on rocks)
- Black or brown (tea colored) water
- Filamentous algae
Biological Effects
- Damage to gill epithelium
- Mucus on gills
- Decreased growth
- Reproductive failure
- Respiratory inhibition
- Ionoregulatory impacts
- Reduced number of species and individuals
- Mortality
- Replacement of acid-sensitive species with acid-tolerant species
Consider contributing, modifying, and related factors when listing low pH as a candidate cause:
- Metals: Common interactions to consider with low pH involve metals (e.g., aluminum, copper, zinc). Acid rain, for example, tends to mobilize and leach metals such as aluminum into groundwater and to streams resulting in higher dissolved metal concentrations in streams combined with low pH. Metals such as aluminum become increasingly bioavailable with decreasing pH (<6.0) due to increases in the free ionic form, which results in greater toxic effects at low pH than at neutral or high pH (Howells et al. 1983, Playle et al. 1989). Depending on the concentration of metal present and how low the pH, pH may be the dominant direct cause of biological effects (with metals being contributors to the effect), or metals may be the dominant direct cause of effects (with pH being a contributor), or the combination of the two may be the cause (i.e., neither acting alone would have caused observed effects).
- Ionic strength: To a large extent, pH controls both the chemical form and the solubility of ionic chemicals.
- Unspecified toxic chemicals: The toxicity of compounds, such as phenols and cyanides, may increase with decreasing pH. For example, six chloro-, bromo-, and nitro-substituted phenols were more toxic to the guppy Poecilia reticulate at a pH of 5 or 6 than at a pH of 7 or 8 (Saarikoski 1981). Also, hydrogen cyanide (HCN) predominates at low and middle pH values and is about twice as toxic as the ion (CN-), which is found in appreciable amounts above pH 8.5 (Rand 1995).
- Ammonia: The concentration and resulting toxicity of NH3 increases as pH increases, although less NH3 is required to produce toxic effects at lower pH (IPCS 1986, Wurts 2003).
High pH

A waterbody is considered to have a high pH, if pH exceeds 9 for prolonged periods of time or with high frequency. High pH is less common than low pH as a candidate cause because anthropogenic sources are acidic more often than basic. High pH is addressed in this module as a proximate cause of effects on aquatic biota. It should be listed as a candidate cause when potential human sources and activities, site observations, or observed biological effects support portions of the source-to-impairment pathways in the conceptual diagram for high pH (Figure 3). This diagram and some of the other information also may be useful in Step 3: Evaluate Data from the Case.
Checklist of sources, site evidence and biological effects
The checklist below will help you identify key data and information useful for determining whether to include high pH among your candidate causes. The list is intended to guide you in collecting evidence to support, weaken, or eliminate high pH as a candidate cause. For more information on specific sources and activities, site evidence, and biological effects listed in the checklist, click on checklist headings or go to the When to List tab of this module.
Consider listing high pH as a candidate cause when the following sources and activities, site evidence, and biological effects are present:
Sources and Activities
- Industrial discharges
- Alkaline geology and soils
- Asphalt production or disposal
- Agricultural lime
- Oil and gas brines
- Industrial landfills
- Cement manufacturing
- Soap manufacturing
- Limestone gravel roads
Site Evidence
- Abundant macrophytes, filamentous algae, or algal mats
- High site pH data
- Mineral deposits
- Low nighttime or early morning dissolved oxygen concentration
(e.g., <40% saturation)
Biological Effects
- Decreased reproduction
- Reduced biodiversity
- Decreased growth
- Damage to skin, gills, olfactory organs, eyes
Consider contributing, modifying, and related factors when high pH is selected as a candidate cause:
- Dissolved oxygen
- Ionic strength: To a large extent, pH controls both the chemical form and the solubility of ionic chemicals.
- Ammonia: The concentration and resulting toxicity of NH3 increases as pH increases, although less NH3 is required to produce toxic effects at lower pH (IPCS 1986, Wurts 2003).
