Eligibility, Medical History, and Deferral Information
Eligibility
Only 38% of the general population are eligible to donate, but fewer than 10% of those eligible actually donate.
Please use the information on this site as a general guide; an evaluation by a medical professional is the only way to determine eligibility.
- do not skip meals
- be sure to drink plenty of fluids (water/juices)
- bring photo identification
Donors must:
- be at least 17 years of age (by California law, all 16 year olds must have a parent's or legal guardian's permission to give a community blood donation by bringing a completed Stanford Blood Center Parental/Guardian consent for 16 year olds form with them to register to donate. Download Form 05-FX1 Consent for Minor to Donate.)
- this form can also be obtained at a Stanford Blood Center donor center, Stanford Blood Center community blood drive, or from your high school blood drive coordinator
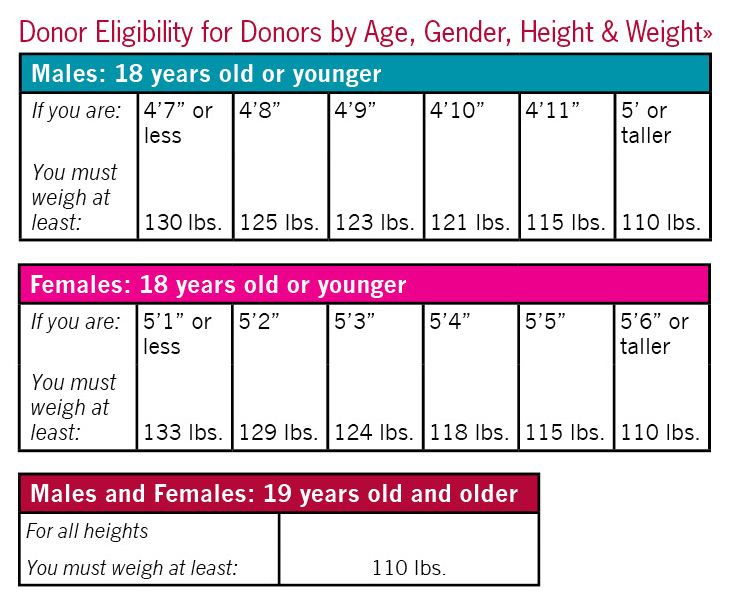
- weigh at least 110 pounds for donors 19 or older (Donors under 19-see height/weight requirements)
- be free of cold and flu symptoms (allergies ok; as are most medications)
- eat before donating and drink plenty of fluids
- bring photo ID
- fill out a Medical History Questionnaire (which will be provided at the time of your donation) and discuss answers confidentially with a Medical Historian
Because blood is a living tissue that is transplanted into another human, the U.S Food and Drug Administration (FDA), in an attempt to ensure a safe blood supply, has imposed strict controls on who may donate. If you discover that you are not eligible to give blood, you can still save lives by providing blood for research, coordinating a blood drive within your organization, volunteering your time, or contributing financially. However you participate in our programs, you are helping maintain the health of our community. Thank you for your dedication.
After viewing the Medical History Form and the common reasons for deferral below, if you have questions about your eligibility, contact us at (650) 723-7831.
Medical History
You will be asked to complete a Medical History Questionnaire each time you donate blood. Your honesty in answering these questions is a crucial part of the blood donation process.
Once you have completed the form, a Medical Historian will go over your questions and answers with you. He or she may ask for further information, and you will have the opportunity to ask any questions you may have. The Medical Historian will then determine, based on your answers, if you are eligible to donate blood that day. Here are some reasons why a donor may be permanently deferred:
| HIV/AIDS | You are a person with symptoms or laboratory evidence of HIV virus. |
| Cancer | You have had Leukemia, Lymphoma, multiple myeloma and all other hematologic malignancies. |
| Heart Disease | You've ever experienced heart failure or coronary artery disease. Other heart conditions may require your doctor's permission. |
| Hepatitis | You have a history of the disease after the age of 10 |
| Organ Failure | You have experienced kidney, lung, or liver failure. |
Deferral Information
Some people are very disappointed to find that they are not eligible to give blood. There are several reasons for—and even different types of—deferrals. Depending upon the reason, a deferral may be either temporary or permanent. Please read below for more information about some of the common reasons for deferral: hemoglobin, Zika Virus, travel to the Caribbean, travel to a Malaria region, travel to a Creutzfeldt-Jakob Disease region, HIV Exposure, and medications.
If you have any questions or concerns, please contact Stanford Blood Center at (650) 723-7831.
Hemoglobin (temporary deferral, until improved)
During the Medical History part of your donation process, the Historian will take a small blood sample from your finger to test your hemoglobin, or red blood cell level. In order to get an accurate sample of your hemoglobin level, your hands must be warm. Try rubbing your hands together, holding a warm drink, or running warm water over your hands before the finger stick. If your hands are cold, notify a staff member. If your hemoglobin is too low, you will not be able to donate blood that day. Please come back soon!
Click here for information about how to raise your hemoglobin level.
Travel to areas with active transmission of Zika virus, or sexual contact with a man who recently traveled to an active area or was diagnosed with Zika virus infection, may put you at risk of contracting this infection. Although a donor may feel well and healthy at the time of donation, a donor with risk factors may be infected with Zika virus and not experience any symptoms. Please click on the link below to learn more about Zika virus, ways one can be exposed to this virus, and to see the current list of areas that have active Zika virus transmission.
bloodcenter.stanford.edu/donate/zika.php
Blood Donors with Recent Travel to the Caribbean
Travel to the Caribbean islands may put you at risk of contracting infections not currently found in the United States that can be transmitted to patients undergoing blood transfusions. Of most concern at this time is a tropical disease caused by chikungunya virus, which is transmitted to humans by infected mosquitoes. Although you report feeling completely well and healthy at the time of your donation, you may still have been infected with chikungunya virus during your travel and not experience any symptoms. Because of the risk of chikungunya virus, donors who have traveled to any island in the Caribbean during the two weeks before donating should notify us as soon as possible at 1-650-725-9968 if they
- become ill within 24 hours after donating;
- develop any symptoms within two (2) weeks of your donation and have no specific diagnosis; or
- are diagnosed by a physician as having Chikungunya infection.
By doing so, you will assist us in preventing the potential for this virus to be transmitted to those receiving blood transfusions.
More information provided by CDC is available for download
Travel to a Malarial region (one-year temporary deferral)
Because of the risk of Malaria, donors who have traveled to certain countries may be deferred from donating blood for one year. If you have traveled to India, you must wait one year from your return to donate.
Certain parts of Mexico, China, Africa, South and Central America, the Caribbean, and the Philippines are considered "limited risk areas". For the most current information available, see the Centers for Disease Control's (CDC) Malaria Risk Map. (Malaria risk changes over time with rainfall patterns or successes in malaria control efforts, and updates to the CDC's Malaria risk map are being made constantly.)
Residence in a Creutzfeldt-Jakob Disease region (lifetime deferral)
Because of the risk of variant Creutzfeldt-Jakob Disease (vCJD) (also known as Mad-Cow Disease) and other blood-related illnesses, some donors may be deferred for one year or permanently because of their travel history. For more information about this deferral, please see the FDA Q&A site or the FDA Blood Guidance site about vCJD.
Individuals who have spent three cumulative months or more in the U.K. between 1980 and 1996 are indefinitely deferred. Also, individuals who spent five years or more since 1980 in some European countries became ineligible as well. The FDA is hoping to eliminate about 90% of the theoretical transmission risk of vCJD with these tightened restrictions.
The following individuals are ineligible to donate blood for transfusion to others:
- Individuals who spent a total of 3 months or more in the United Kingdom from 1980-1996.
- Individuals who were in the U.S. military, dependents of U.S. military, or civilian military and were stationed in certain European countries for 6 months or more between 1980-1996. (This is because some U.S. military bases in Europe obtained their meat from the United Kingdom. Please contact us to discuss where you were stationed.)
- Individuals who lived in France for 5 years or more since 1980.
- Individuals who received a blood transfusion in the United Kingdom or France since 1980.
- Individuals who spent 5 years or more since 1980 in some other
European countries (please contact our staff to discuss your situation).
| England | Wales | Gibraltar |
| Northern Ireland | Isle of Man | Channel Islands |
| Scotland | Falkland Islands |
| Albania Austria Belgium Bosnia-Herzegovina Bulgaria Croatia Czech Republic Denmark |
Finland France Germany Greece Hungary Republic of Ireland Italy Liechtenstein |
Luxembourg Macedonia Netherlands Norway Poland Portugal Romania Slovak Republic |
Slovenia Spain (including: The Canary Islands Penon de Velez de La Gomera, Penon De Ahucemas, Islas Chafarinas, Centa and Melilla) Sweden Switzerland United Kingdom Federal Republic of Yugoslavia(Kosovo, Montenegro Serbia, Yugoslavia) |
HIV Exposure (lifetime or one-year deferral)
The FDA requires all blood centers to question donors about activities that are associated with an increased risk of exposure to infectious agents. According to statistics from the public health service, men who have sex with men continue to represent a population at increased risk of acquiring HIV infection. Although heterosexual spread of HIV is increasing nationally, in California men who have sex with men continue to account for approximately 2/3 of HIV infections. Men who have had sex with a man are permanently not eligible to donate blood. Women who have had sex with a man who has had sex with a man are not eligible to donate blood for one year. For more information on this deferral, please see our Explaining the MSM Deferral page.
Medications
Certain medications that are perfectly safe for you to take could be harmful if transfused into another person. If you are currently taking or have ever taken any of the medications listed below, you should let our staff know. The deferral periods for these medications vary; your Medical Historian will discuss your eligibility with you.
- Accutane© (Absorica, Amnesteem, Claravis, Myorisan, Sotret, Zenatane [isotretinoin])
- Anticoagulants or "blood thinner," such as:
- Coumadin, Warfilone, Jantoven – (warfarins)
- Heparin or Low Molecular Weight Heparins
- Arixtra (fondaparinux)
- Lovenox (enoxaparin)
- Pradaxa (dabigatran)
- Direct Xa Inhibitors ("xabans" e.g. rivaroxaban, apixaban, endoxaban)
- Anti-platelet agents, such as:
- Plavix (clopidogrel)
- Ticlid (ticlopidine)
- Effient (prasugrel)
- Brilinta (ticagrelor)
- Avodart©, Jalyn (dutasteride)
- Experimental Medication or Unlicensed (Experimental) Vaccine; usually associated with a research protocol
- Feldene
- Growth Hormone from Human Pituitary Glands
- Hepatitis B Immune Globulin (this is different from the hepatitis B vaccine which is a series of 3 injections given over a 6 month period to prevent future infection from exposures to hepatitis B)
- Insulin from Cows (Bovine, or Beef, Insulin)
- Propecia© (finasteride)
- Proscar© (finasteride)
- Soriatane© (acitretin)
- Tegison© (etretinate)
