Division News
Excerpts from the Stanford medical community
The Health Trust hosts the 3rd Annual World AIDS Day Benefit Dinner
The event is a benefit for the Health Trust AIDS Services program and honors professionals for their service to individuals with HIV in the community.
This year, they honored Dr. Larry Mc Glynn with the Red Ribbon Award for outstanding service to individuals with HIV/AIDS. The Health Trust AIDS Services staff and many community partners in the field acknowledge Dr. Mc Glynn's expertise, compassion and longstanding commitment to people with HIV/AIDS in our community.
Dr. Mc Glynn is a Clinical Professor in the Department of Psychiatry and Behavioral Sciences at Stanford University. Since 2000, he has served as the Director of the HIV Mental Health Program at Stanford's Positive Care Clinic.
Researchers identify biomarkers associated with chronic fatigue syndrome severity
Stanford investigators used high-throughput analysis to link inflammation to chronic fatigue syndrome, a difficult-to-diagnose disease with no known cure.

Jose Montoya and his colleagues have found evidence inflammation may be the culprit behind chronic fatigue syndrome, a disease with no known cure.
Steve Fisch
Researchers at the Stanford University School of Medicine have linked chronic fatigue syndrome to variations in 17 immune-system signaling proteins, or cytokines, whose concentrations in the blood correlate with the disease’s severity.
The findings provide evidence that inflammation is a powerful driver of this mysterious condition, whose underpinnings have eluded researchers for 35 years.
The findings, described in a study published online July 31 in the Proceedings of the National Academy of Sciences, could lead to further understanding of this condition and be used to improve the diagnosis and treatment of the disorder, which has been notably difficult.
More than 1 million people in the United States suffer from chronic fatigue syndrome, also known as myalgic encephomyelitis and designated by the acronym ME/CFS. It is a disease with no known cure or even reliably effective treatments. Three of every four ME/CFS patients are women, for reasons that are not understood. It characteristically arises in two major waves: among adolescents between the ages of 15 and 20, and in adults between 30 and 35. The condition typically persists for decades.
“Chronic fatigue syndrome can turn a life of productive activity into one of dependency and desolation,” said Jose Montoya, MD, professor of infectious diseases, who is the study’s lead author. Some spontaneous recoveries occur during the first year, he said, but rarely after the condition has persisted more than five years.
The study’s senior author is Mark Davis, PhD, professor of immunology and microbiology and director of Stanford’s Institute for Immunity, Transplantation and Infection.
‘Solid basis for a diagnostic blood test’
“There’s been a great deal of controversy and confusion surrounding ME/CFS — even whether it is an actual disease,” said Davis. “Our findings show clearly that it’s an inflammatory disease and provide a solid basis for a diagnostic blood test.”
Many, but not all, ME/CFS patients experience flulike symptoms common in inflammation-driven diseases, Montoya said. But because its symptoms are so diffuse —sometimes manifesting as heart problems, sometimes as mental impairment nicknamed “brain fog,” other times as indigestion, diarrhea, constipation, muscle pain, tender lymph nodes and so forth — it often goes undiagnosed, even among patients who’ve visited a half-dozen or more different specialists in an effort to determine what’s wrong with them.

Mark Davis
Montoya, who oversees the Stanford ME/CFS Initiative, came across his first ME/CFS patient in 2004, an experience he said he’s never forgotten.
“I have seen the horrors of this disease, multiplied by hundreds of patients,” he said. “It’s been observed and talked about for 35 years now, sometimes with the onus of being described as a psychological condition. But chronic fatigue syndrome is by no means a figment of the imagination. This is real.”
Antivirals, anti-inflammatories and immune-modulating drugs have led to symptomatic improvement in some cases, Montoya said. But no single pathogenic agent that can be fingered as the ultimate ME/CFS trigger has yet been isolated, while previous efforts to identify immunological abnormalities behind the disease have met with conflicting and confusing results.
Still, the sporadic effectiveness of antiviral and anti-inflammatory drugs has spurred Montoya to undertake a systematic study to see if the inflammation that’s been a will-o’-the-wisp in those previous searches could be definitively pinned down.
To attack this problem, he called on Davis, who helped create the Human Immune Monitoring Center. Since its inception a decade ago, the center has served as an engine for large-scale, data-intensive immunological analysis of human blood and tissue samples. Directed by study co-author Holden Maecker, PhD, a professor of microbiology and immunology, the center is equipped to rapidly assess gene variations and activity levels, frequencies of numerous immune cell types, blood concentrations of scores of immune proteins, activation states of intercellular signaling models, and more on a massive scale.
Finding patterns
This approach is akin to being able to look for and find larger patterns — analogous to whole words or sentences — in order to locate a desired paragraph in a lengthy manuscript, rather than just try to locate it by counting the number of times in which the letter A appears in every paragraph.
The scientists analyzed blood samples from 192 of Montoya’s patients, as well as from 392 healthy control subjects. The average age of patients and controls was about 50. Patients’ average duration of symptoms was somewhat more than 10 years.
Importantly, the study design took into account patients’ disease severity and duration. The scientists found that some cytokine levels were lower in patients with mild forms of ME/CFS than in the control subjects, but elevated in ME/CFS patients with relatively severe manifestations. Averaging the results for patients versus controls with respect to these measures would have obscured this phenomenon, which Montoya said he thinks may reflect different genetic predispositions, among patients, to progress to mild versus severe disease.
I have seen the horrors of this disease, multiplied by hundreds of patients.
I have seen the horrors of this disease, multiplied by hundreds of patients.
When comparing patients versus control subjects, the researchers found that only two of the 51 cytokines they measured were different. Tumor growth factor beta was higher and resistin was lower in ME/CFS patients. However, the investigators found that the concentrations of 17 of the cytokines tracked disease severity. Thirteen of those 17 cytokines are pro-inflammatory.
TGF-beta is often thought of as an anti-inflammatory rather than a pro-inflammatory cytokine. But it’s known to take on a pro-inflammatory character in some cases, including certain cancers. ME/CFS patients have a higher than normal incidence of lymphoma, and Montoya speculated that TGF-beta’s elevation in ME/CFS patients could turn out to be a link.
One of the cytokines whose levels corresponded to disease severity, leptin, is secreted by fat tissue. Best known as a satiety reporter that tells the brain when somebody’s stomach is full, leptin is also an active pro-inflammatory substance. Generally, leptin is more abundant in women’s blood than in men’s, which could throw light on why more women than men have ME/CFS.
More generally speaking, the study’s results hold implications for the design of future studies of disease, including clinical trials testing immunomodulatory drugs’ potential as ME/CFS therapies.
“For decades, the ‘case vs. healthy controls’ study design has served well to advance our understanding of many diseases,” Montoya said. “However, it’s possible that for certain pathologies in humans, analysis by disease severity or duration would be likely to provide further insights.”
Other Stanford co-authors of the study are clinical research coordinator Jill Anderson; Tyson Holmes, PhD, senior research engineer at the Institute for Immunity, Transplantation and Infection; Yael Rosenberg-Hasson, PhD, immunoassay and technical director at the institute; Cristina Tato, PhD, MPH, research and science analyst at the institute; former study coordinator Ian Valencia; and Lily Chu, MSHS, a board member of the Stanford University ME/CFS Initiative.
The study was funded by the National Institutes of Health (grant U19AI057229), the Stanford ME/CFS Initiative Fund and an anonymous donor.
Stanford’s departments of Medicine and of Microbiology and Immunology also supported the work.
By Bruce Goldman via Stanford Medicine News Center
Dr. Dean Winslow receives the IDSA Society Citation
Dean Winslow, MD, FIDSA
Society Citation Dean L. Winslow, MD, FIDSA, a compassionate, committed clinician, teacher, and military veteran who has been a champion for patients with HIV and victims of war and disaster, is the recipient of a 2017 IDSA Society Citation. First awarded in 1977, this is a discretionary award given in recognition of exemplary contribution to IDSA, an outstanding discovery in the field of infectious diseases, or a lifetime of outstanding achievement.
Dr. Winslow is vice chair of the Department of Medicine and a professor of medicine in the Division of Hospital Medicine and the Division of Infectious Diseases and Geographic Medicine at Stanford University, where he has been on the faculty since 1998. He is also academic physician-in-chief at Stanford/ValleyCare, a community teaching hospital. His professional career began in private practice in Delaware, where he started the state’s first multidisciplinary clinic for HIV-infected patients in 1985. During his later time in industry, he worked on studies of HIV drug resistance as a bench scientist and designed the clinical trials leading to the Food and Drug Administration’s approval of efavirenz. He also helped direct the clinical studies of nelfinavir and led the group responsible for regulatory approval of the first pharmacogenomics diagnostic device for HIV-1 drug resistance.
Before returning to Stanford fulltime in 2013, Dr. Winslow was chief of the Division of AIDS Medicine and chair of the Department of Medicine at Santa Clara Valley Medical Center, the institution’s largest academic and clinical unit. In addition to caring for adults, Dr. Winslow regularly attended on the pediatric infectious diseases consult service at the county hospital. From 2003 to 2011, Dr. Winslow also deployed to Iraq and Afghanistan six times as a flight surgeon in the Air National Guard in support of combat operations there, including serving as the commander of an Air Force combat hospital unit in Baghdad in 2008. Closer to home, he coordinated military public health and force protection in Louisiana after Hurricane Katrina. He has been awarded numerous military decorations for his service.
In addition to his care for wounded service personnel in the Middle East, Dr. Winslow also treated many local civilians. A passionate supporter of human rights and health, he has, since 2006, arranged for medical care, transportation, and housing in the U.S. for more than 20 Iraqi children and adults with complicated medical conditions for which surgical care was not available in Iraq. He has been recognized by the Iraqi Army for his humanitarian service to the Iraqi people and was named a Paul Harris Fellow by Rotary International.
After earning his medical degree from Jefferson Medical College in Philadelphia, Dr. Winslow completed his internship and residency in internal medicine at the Medical Center of Delaware, followed by a fellowship in infectious diseases at the Ochsner Clinic in New Orleans. A highly regarded attending physician on the ID consultation services at Stanford and the Palo Alto Veterans Administration Medical Center, Dr. Winslow has received multiple teaching awards. His wide-ranging knowledge and experience, combined with his passion for teaching and patient care, are legendary among ID fellows and residents. The author of more than 60 peer- reviewed publications and more than 90 presentations at national and international meetings, he is the current chair of IDSA’s Standards and Practice Guidelines Committee.
Dr. Winslow’s extensive knowledge, deep compassion, and wide-ranging experience over more than four decades have greatly impacted patients around the world, his colleagues, and the next generation of ID physicians. The Society is delighted to add a 2017 Society Citation to his long list of accomplishments
Paul Bollyky Receives Harrington Scholar-Innovator Grant
Source: Department of Medicine News
Harrington Discovery Institute has selected Paul Bollyky, MD, (assistant professor, infectious diseases) as one of its 2017 Scholar-Innovators. Chosen for his work on a novel drug for Type I Diabetes, Bollyky is one of 11 awardees this year.
The Institute grants each recipient a minimum of $100,000 (along with its drug development expertise and project management support) for breakthrough discoveries defined by innovation, creativity and potential clinical impact.
Bollyky says the award is “designed to accelerate breakthrough discoveries into new medicines” and adds, “In our case, this work will fund development of a novel therapeutic to prevent autoimmune diabetes.” Noting how innovative the research is, Upi Singh, MD, (chief, infectious diseases) says, “An infectious disease (ID) doc doing diabetes research! As I always say, everything is really ID. [This is] a nice example that science boundaries really are blurring and the best science is done at the interface of multiple disciplines.”
Throughout his career, Bollyky has earned a number of awards and grants, including a Catalyst Award from the Dr. Ralph and Marian Falk Medical Research Trust, the Outstanding Faculty Mentor Award, a SPARK Innovation Grant, a seed grant from Stanford Institute for Immunity, Transplantation and Infection and a Career Development Award from the Juvenile Diabetes Research Foundation.
Bollyky attended college at Columbia University in New York City. He did his Ph.D. at Oxford University and went to Harvard Medical School for his M.D. He completed his residency at Brigham and Women’s Hospital, an infectious disease fellowship at the University of Washington and a post-doctoral fellowship at the Benaroya Research Institute. He was appointed to the Stanford faculty in 2013.
Drug combination defeats dengue, Ebola in mice
Source: Stanford News Center
By Bruce Goldman
To develop a potential antiviral treatment, Stanford researchers adopted an unusual approach: Rather than trying to disable viral enzymes, they targeted proteins the infected individual makes — and the virus needs.
A combination of two cancer drugs inhibited both dengue and Ebola virus infections in mice in a study led by Stanford University School of Medicine researchers, despite the fact that these two viruses are vastly different from each other.
In laboratory-dish experiments, the drug combination, which has previously shown efficacy against the hepatitis C virus, also was effective against West Nile and Zika viruses, both of which are relatives of the hepatitis C virus, and multiple other unrelated viruses.
The multi-institution study, published online Feb. 27 in the Journal of Clinical Investigation, also pinpointed the specific molecular mechanism by which these drugs derail a variety of RNA viruses, whose genetic material consists not of DNA but of its close relative, RNA.
“We’ve shown that a single combination of drugs can be effective across a broad range of viruses — even when those viruses hail from widely separated branches of the evolutionary tree,” said the study’s senior author, Shirit Einav, MD, assistant professor of infectious diseases and of microbiology and immunology.
The study’s lead authors are former Stanford postdoctoral scholars Elena Bekerman, PhD, now at Gilead Sciences Inc., and Gregory Neveu, PhD, now at the University of Lyon and French National Institute of Health and Medical Research.
The reason the drugs used in the study are able to combat infections by such different viruses is that their disabling action is directed not at the virus but at proteins of the host cell it’s trying to infect, Einav said.
The challenges posed by RNA viruses
Einav and her team are investigating strategies for combatting RNA viruses, such as dengue and Ebola. These viruses have a faulty replication process that results in frequent errors as their genetic material is copied, rendering them especially prone to mutations. Consequently, they swiftly acquire resistance to a typical antiviral drug that targets a specific viral enzyme, Einav said.
We’ve shown that a single combination of drugs can be effective across a broad range of viruses.
“The ‘one drug, one bug’ approach can be quite successful, as in the case of hepatitis C virus,” for which a concerted effort has generated several approved antiviral treatments, she said. But it took more than 10 years of research, she noted, and drug development costs typically exceed $2 billion. Making matters worse, Einav added, is the impossibility of predicting what the next emerging viral threat will look like.
“We’re always getting blindsided,” she said.
The deadly Ebola epidemic of a few years ago has subsided but could return at any time. Dengue infects an estimated 390 million people annually in over 100 countries. Four distinct strains of the dengue virus exist, hampering the development of a vaccine and boosting the chances of a once-infected person’s re-infection by a different strain against which that person hasn’t achieved sufficient immunity. Secondary infections can become life-threatening.
While an Ebola vaccine has shown promise, it’s not yet approved. A recently approved dengue vaccine has only limited efficacy. No viable antiviral drugs are currently available for either virus.
We’ve shown that a single combination of drugs can be effective across a broad range of viruses — even when those viruses hail from widely separated branches of the evolutionary tree
Picking a different target
Viruses are cut-rate brigands: They produce nothing on their own, but rather hijack the machinery of our cells. Hepatitis C, dengue, Ebola and other viruses hop onto molecular “buses” that whisk cargo between cell compartments. These buses shuttle the viruses around inside of cells. The buses’ routes and fares are regulated by numerous cellular enzymes. Two such enzymes, which go by the acronyms AAK1 and GAK, essentially lower the fares charged by the molecular buses by tweaking them so they bind more strongly to their cargo.
The standard antiviral approach aims to disable a specific viral enzyme. Einav and her associates’ alternative approach took advantage of viruses’ total dependence on infected cells’ molecular machinery.
The two-drug drug combination Einav’s team put to work against dengue and Ebola impedes AAK1’s and GAK’s activity, effectively pricing bus fares beyond the viral budget. Erlotinib and sunitinib, each approved by the Food and Drug Administration more than a decade ago, are prescribed for various cancer indications. Neither AAK1 nor GAK are the primary targets of these drugs in their cancer-fighting roles. But Einav’s group discovered, by accessing publicly available databases, that the two drugs impair AAK1 and GAK activity, too.
Einav and her colleagues previously demonstrated that erlotinib and sunitinib inhibit hepatitis C virus infection in cells. In the new study, the investigators conducted experiments in lab dishes to show that both drugs inhibit viral infection by impeding the activity of AAK1 and GAK.
Next, they tested the combination in lab dishes against the dengue and Ebola viruses, and observed that viral activity was strongly inhibited in both. While the dengue virus is a relatively close cousin of hepatitis C, it is quite different from the Ebola virus. The same drug combination also showed efficacy against a variety of other RNA viruses related to hepatitis C, including the Zika and West Nile viruses, and even against several unrelated viruses.
Testing the combination in mice
In a prevention experiment in mice, the investigators administered the erlotinib-sunitinib combination once daily starting on the day of dengue-virus infection, employing the two drugs for five days at doses comparable to those approved for use against cancer in humans. All the control mice died between days four and eight. But of those treated with the drug combination, 65 to 100 percent, depending on the individual experiment, survived and regained their pre-infection weight and mobility. Given individually, the drugs provided substantially less protection, Einav said.
In another experiment designed to test the drugs as a therapy, the combination retained substantial antiviral efficacy as long as it was given less than 48 hours after infection.
In a similar prevention experiment with the Ebola virus, the scientists administered the drug daily for 10 days starting at six hours before infection. Some 90 percent of the control mice died within a week or two. But half the mice receiving the drug combination survived. Again, the drugs were substantially less effective when given individually.
Additional lab experiments showed that the combination profoundly inhibited the dengue virus’s ability to develop drug resistance. There’s no possible way for viral mutations to alter the proteins of the cells it infects, Einav said, and no easy way for the virus to mutate around its dependence on those proteins.
Stanford’s Office of Technology Licensing has filed for patents on intellectual property associated with the findings.
Other Stanford study co-authors are Claude Nagamine, DVM, PhD, assistant professor of comparative medicine; and research scientist Robert Mateo, PhD.
The study was carried out in collaboration with researchers from the University of Chicago, the U.S. Army Medical Research Institute of Infectious Diseases in Maryland, the Washington University School of Medicine in St. Louis and the University of Leuven in Belgium.
The study was funded by the National Institute of Health (grants IU19AI10966201 and U19A1083019); the American Cancer Society; the Doris Duke Charitable Foundation; the Department of Defense; Stanford Bio-X; the Stanford SPARK program; the Stanford Translational Research and Applied Medicine program; Spectrum, which administers Stanford’s Clinical and Translational Science Award (grant UL1TR001085) from the NIH; the Stanford Child Health Research Institute; and the Taiwan Ministry of Science and Technology.
Stanford’s departments of Medicine and of Microbiology and Immunology also supported the work.
15 School of Medicine researchers named CZ Biohub investigators
Source: Stanford Medicine News
Photo Courtesy: Chan Zuckerberg Biohub
The researchers will be given funding by the Chan Zuckerberg Biohub to develop tools and technologies that support the organization’s goal of curing, preventing or managing every disease.
Fifteen faculty members from the School of Medicine are among the 47 investigators announced today by the Chan Zuckerberg Biohub.
The CZ Biohub is an independent nonprofit medical research organization that has the goal of harnessing the power of science, technology and human capacity to cure, prevent or manage all disease. It is funded through a $600 million commitment by the Chan Zuckerberg Initiative, which was created by Facebook founder Mark Zuckerberg and his wife Priscilla Chan, MD.
The investigators were selected from the three institutions participating in the CZ Biohub: Stanford, UC-San Francisco and UC-Berkeley. Each of the investigators will be given a five-year appointment and up to $1.5 million for research in their respective areas of expertise. More than 700 researchers applied for the funding; the selections were made by an international panel of 60 scientists and engineers.
The investigators include both senior researchers and up-and-coming faculty.
“The 47 CZ Biohub investigators we’re introducing today are quite literally inventing the future of life science research,” said Stephen Quake, PhD, co-president of CZ Biohub and professor of bioengineering and applied physics at Stanford. “The CZ Biohub is distinguished by our emphasis on technology and engineering, and our researchers are inventing tools to accelerate science for the good of humanity.”
“We are honored to have so many of our scientists selected to pursue their innovative and ambitious projects at the Chan Zuckerberg Biohub,” said Lloyd Minor, MD, dean of the School of Medicine. “If past is prologue, giving such inventive thinkers the freedom to conduct fundamental research will result in truly outstanding discoveries, moving us toward a future where we can both cure and prevent what today seems incurable and unpredictable.”
We are honored to have so many of our scientists selected to pursue their innovative and ambitious projects
The 15 medical school faculty members are:
Senior investigators
- Carlos Bustamante, PhD, professor of biomedical data science and of genetics: He is focusing on the integration and analysis of massive data coming from consumer, health care and financial sources. He is especially interested in bringing together direct-to-consumer genetics and phenotype data in a secure space that can be explored by academic-, industry- and citizen-scientists.
- Brian Kobilka, MD, professor of molecular and cellular physiology: His pioneering X-ray crystallographic studies have revealed how the binding of a hormone to the extracellular pocket on a G-protein coupled receptor is transmitted across the cell membrane to trigger a signaling cascade. He is now carrying out structural studies of opioid receptors to identify more effective painkillers with fewer side effects.
- Matthew Porteus, MD, PhD, associate professor of pediatrics: He uses genome editing as curative therapy for genetic diseases, as exemplified by his correction of the mutation in sickle cell disease in hematopoietic stem and progenitor cells. He is now combining genome editing with synthetic biology to engineer cells having new phenotypic properties, such as resistance to HIV and enhanced wound healing.
- Lucy Shapiro, PhD, professor of developmental biology: She has established the bacterium Caulobacter cresentus as a powerful model organism for understanding self-organization and spatially controlled differentiation leading to daughter cells with different cell fates. She is developing a reaction-diffusion model that includes all essential cellular processes to gain a deeper understanding of asymmetric cell division and cell polarity.
- Christina Smolke, PhD, professor of bioengineering: She is engineering yeast to produce complex, plant-inspired medicinal compounds like those widely used as antihypertensives and anticancer agents. She interacts with experts in plant-specialized metabolism to identify gene clusters that can be inserted into her optimized yeast platform to accelerate the discovery of new therapeutic agents.
- Tom Soh, PhD, professor of radiology and of electrical engineering: He has devised sensors capable of continuously monitoring specific biomolecules in vivo and a control system for achieving real-time, closed-loop controlled drug delivery in live animals. He plans to generate detection systems for hitherto untargetable biomolecules and to develop real-time sensors that can be implanted in vivo to detect multiple biomolecules that are medically important.
- Alice Ting, PhD, professor of genetics and of biology: She develops, scales up and broadly disseminates molecular technologies for mapping cells and functional circuits, as illustrated by her biotin-based method for protein mapping in living cells. She is devising methods for identifying the ensemble of neurons that encode or control a specific memory, behavior or emotional state by using a light- and calcium-gated transcription factor.
Junior investigators
- Catherine Blish, MD, PhD, assistant professor of infectious diseases: She aims to build an atlas of host-pathogen interactions to serve as a template to elicit immune responses that will promote pathogen eradication. She seeks to understand how to control the innate immune response mediated by natural-killer and other cells to eliminate infections and develop more potent methods of protection.
- Adam de la Zerda, PhD, assistant professor of structural biology: His goal is to image 100 million cells in living tissues at single-cell resolution by using optical coherence tomography. One of the potential uses of his technique will be to visualize cancer markers to delineate the margins of tumors.
- Polly Fordyce, PhD, assistant professor of genetics and of bioengineering: She is developing new biochip technologies for high-throughput functional characterization of proteins to enhance our ability to predict the function of a protein given its amino acid sequence. Her aim is to characterize the properties of more than 1,000 proteins, such as enzymes and transcription factors, in a single experiment.
- William Greenleaf, PhD, assistant professor of genetics: He studies the physical and spatial organization of the human genome at multiple scales and across different biological states. His aim is to unravel the quantitative relations between regulatory elements and gene expression in a massive, parallel way to generate a quantitative model of the regulatory wiring of cells.
- Manu Prakash, PhD, assistant professor of bioengineering: He develops measurement tools, such as ultra-low cost microscopy platforms for field diagnostics of infectious diseases, for use in resource-poor areas of the world. His aim is to devise new, frugal platforms for the diagnosis and surveillance of schistosomiasis, leishmaniasis and malaria.
- Taia Wang, MD, PhD, assistant professor of infectious diseases: She studies human immunity and susceptibility to viral pathogens such as dengue virus. Her research is driven by the finding that humans have diverse immunoglobulin Fc domains that affect the severity of viral diseases and the effectiveness of vaccines.
- Ellen Yeh, MD, PhD, assistant professor of biochemistry, of pathology and of microbiology and immunology: She studies the apicoplast, a unique organelle in Plasmodium falciparum parasites, to identify new targets for the prevention and therapy of malaria. She aims to comprehensively identify the apicoplast proteome and to understand the novel secretory pathways of this unusual plastid in her search for novel therapeutic targets.
- James Zou, PhD, assistant professor of biomedical data science: He develops novel machine-learning tools that enable researchers to make complex predictions and quantify disease mechanisms using population genomics and epigenomics data. He is devising new deep-learning models to increase the accuracy of predicting genetic risk from genotypes and of identifying distinct cell populations based on single-cell transcriptional profiles.
Additionally, four Stanford faculty members from other schools were also named CZ Biohub investigators, along with 15 researchers from UCSF and 13 from UC-Berkeley.
5 Questions: Taia Wang on why some develop severe dengue disease
Written by Ruthann Richter
Source: Stanford Medicine News
A new study has found a specific immunologic response among people likely to get severe dengue disease. The work could help lead to a screening test for people at risk of getting a serious case of the disease and to targeted vaccines.
Dengue is a mosquito-borne viral infection that can lead to severe disease and death. Endemic in at least 100 countries, it is carried by the same mosquitoes that transmit Zika, yellow fever and chikungunya infections. The incidence of dengue infection has risen sharply in recent years, with some 50 million to 100 million cases reported each year.
Some infected individuals become ill with flulike symptoms, such as high fever, headache, vomiting, muscle and joint pain and skin rash. But in a small number of cases, it leads to more serious symptoms, including severe abdominal pain, persistent vomiting and severe bleeding. As many as 20,000 people die every year of the disease, which has no specific treatment. A novel vaccine for dengue was introduced in late 2015 and is commercially available in some countries. But large-scale trials of the vaccine have shown it to be only about 60 percent effective in people older than 9, and less than 45 percent effective in children younger than that.
Stanford virologist Taia Wang, MD, PhD, an assistant professor of infectious diseases and geographic medicine, was the lead author of a study published Jan. 27 in Science which found that some people may be more susceptible to severe dengue disease than others. Writer Ruthann Richter recently asked Wang about the disease and the study’s findings.
Q: Why is dengue so difficult to prevent and treat?
Wang: Dengue viruses are an especially challenging target from a vaccine development perspective. Most effective vaccines work by producing antibodies, a subset of which can prevent the virus from entering a cell by “neutralizing” it. Unlike other viruses, antibodies that bind to dengue viruses but fail to neutralize it can actually enhance dengue disease. Making matters worse, there are also four major serotypes of dengue viruses, so an effective and safe vaccine would need to elicit neutralizing antibodies against all four types at once.
The treatment of patients who go on to develop dengue disease poses another set of challenges. Severe cases are most successfully managed when patients are caught early and treated in the hospital, yet we don’t currently have a good way to predict who will develop severe disease. In addition, there are no specific medications that can be used to treat dengue disease; we rely on hydration and blood products for management of severe cases, which is very resource-intensive. This is a serious problem since most dengue infections occur in parts of the world where access to medical care is limited.
Q: Who is at greatest risk for the disease and why?
Wang: The greatest risk factor for progression to severe dengue disease is virus infection in the presence of antibodies that bind to the virus, but do not neutralize it. We typically think of antibodies as protecting us from infection; dengue infections pose a rare example of a situation where some antibodies can enhance disease. This antibody-dependent enhancement most commonly occurs when someone is infected for the second time. When this happens, antibodies that were produced during the first exposure can enhance the severity of subsequent infection. Still, progression to severe disease during second infections is relatively rare, and the reason for progression has not been understood, which limits early clinical intervention. Our study was driven by a desire to understand why some people progress to severe disease during secondary dengue infection while others do not.
Q: Can you tell us about the findings?
Wang: Our previous work showed tremendous variation in the type of antibodies produced by individuals. We were particularly interested in regions of antibodies called Fc domains and their receptors because they have been shown to be directly involved in enhancement of dengue disease. We hypothesized that the variability in Fc domains among people might determine progression to severe dengue. We discovered that people who develop the most severe forms of dengue disease do produce a different repertoire of antibodies — they make antibodies with Fc domains that work through a particular Fc receptor called FcR3a. We went on to show that the antibodies with higher affinity for FcR3a are involved in the development of severe dengue disease.
Q: What are the implications for vaccination programs?
Wang: Since people who develop severe dengue disease produce a distinct type of antibody, it may be possible to develop a screening test that identifies patients who are at highest risk for severe disease before they are ever infected. This would be useful for identifying patients who need a higher level of clinical care during dengue infection. Much more work will be required to determine whether such a test is possible.
In addition to the implications for clinical care, our findings may be used to guide vaccine development. Since we found that a specific phenotype of antibody contributes to severe disease, we would want to avoid stimulating production of those antibodies during vaccination. This could potentially be done by using specific vaccine antigens and/or vaccine adjuvants to guide the immune system to produce antibodies that are less likely to enhance disease.
Q: Why not vaccinate everyone in disease-prone areas?
Wang: Vaccinating everyone in areas where dengue is prevalent with a safe, effective vaccine is the surest way to limit dengue disease. However, doing so would be expensive. In areas with limited resources, the next best thing is probably to target vaccination to people at highest risk for severe disease. For this reason, and because it could help guide clinical care of dengue patients, we are investigating inexpensive screening tests that could be used to identify people at highest risk for progression to severe dengue disease.
A most enthusiastic citizen of the university: Dr. Julie Parsonnet
Source: Department of Medicine
A Research Career to Benefit Children and a Life Lived on Campus Among Undergraduates
Julie Parsonnet, MD, professor of infectious diseases and health research and policy, seems most comfortable describing herself as an “enthusiastic citizen of the university.” She explains this descriptor by saying, “I’ve lived on campus most of my time here, and I’ve had neighbors who are in history and in English and in French. I’ve always been interested in the university as an entity, the way it works and the breadth of knowledge here. There are a lot of things going on at the university that I find appealing and interesting. And I’ve always wanted to be engaged.”
Parsonnet has spent most of her time on her day job doing research, beginning in her fellowship at Massachusetts General Hospital when she became interested in a recently discovered organism called Helicobacter pylori. “Like many scientists,” she says, “I started my career with a mistake. I was convinced that Helicobacter was an unimportant colonizer of the stomach, just an organism that lived in humans. So my first studies were designed to see if it really did anything. As I went further and further along, I found to my great surprise that this organism, which is present in about 50 percent of the world’s population, was actually responsible for a lot of different things: stomach cancers, gastric lymphomas and peptic ulcers, among others. We were involved in discovering links between infection with Helicobacter pylori and both cancer and lymphoma, resulting in New England Journal of Medicine publications in 1991 and 1994.”
For some researchers such results provide a line of sight to years and decades of further work elucidating mechanisms, hypothesizing therapies, grinding out hours upon hours of grant writing and manuscript preparation. Parsonnet went in a somewhat different direction. “After that,” she says, “I started to think if H. pylori is in 50 percent of the world’s population, then maybe it’s not all bad. Maybe the reason it lives in so much of the world’s population is because it provides some sort of survival advantage, particularly to children. And we found that it was associated with lower weights in children and might protect against tuberculosis and diarrheal disease. We learned that it protects against another form of cancer, esophageal adenocarcinoma.”
These findings stimulated more thought, eventually prompting a shift away from a focus on just H. pylori to “looking at the totality of microorganisms. After considering that this one organism is but one of many thousands living in the human body, we started to wonder what else is in there that influences many aspects of human health. Are there other signals that might relate infections to things that we don’t traditionally think of as infectious diseases, such as asthma and allergy and obesity? What we work on now is trying to understand how children acquire infection and what it means for their lifetime health.”
“We” is a pronoun Parsonnet uses frequently in conversation, whether she speaks of the people with whom she collaborates in and outside the lab or the students with whom she and her husband (Dean Winslow, MD, professor of general medical disciplines) live as resident fellows in Robinson House.
“What makes me excited about my research is the same sort of thing that makes me excited about living on campus: We work closely with so many fantastically smart people. People in immunology, Scott Boyd, Mark Davis, Kari Nadeau in pediatric allergy, and the March of Dimes project with David Stevenson and Gary Shaw, and with obesity experts like Christopher Gardner and Tom Robinson. It’s just really, really fun.”
Parsonnet’s successes in grant making have given her the opportunity to help other fantastically smart people make their way in a highly competitive world whether through support from her research grants (including her NIH Director’s award) or through National Institutes of Health training grants (T32s). A T32, of which she has been principal investigator for several cycles, funds salaries and tuition for master’s degrees at Stanford for three fellows in infectious diseases each year. Upinder Singh, MD, associate professor and chief of infectious diseases, describes this as “an invaluable addition to our program. Julie’s success in renewing it, in these tough funding times, is a testament to her skills, effort and dedication.”
For the 20 percent of her life that is not spent on research, Parsonnet can quickly provide a laundry list of campus-focused activities past and present. There have been two presidential task forces, one dealing with international global health and the other with graduate education.
Parsonnet describes the graduate education task force as “a great experience because we got to work with people from graduate education all across the campus addressing such issues as what should be the goal for graduate students here, how we increase interdisciplinary engagement across the campus, and how we support our students financially.”
And then there is teaching: “I’ve been teaching undergraduates for 15 or 20 years. I teach epidemiology and infectious diseases, and this year I started teaching a class on how to investigate an outbreak. A lot of students are fascinated by public health and by outbreaks in particular, and we engaged a lot of people from the public health community. It was an inspiring class and I was happy to find quite a number of students begin to reshape their career aspirations toward public health.”
A while back, there was a deanship: “I was the dean for medical education for five years, and I got to meet a lot of medical students as well as undergraduates applying for medical school. I love that part of the university. I just love being around smart, engaged, interested young people and seeing how they frame their careers and maybe helping them a little get where they want to go. I think that sort of explains pretty much my entire career.”
And finally, in her own summary words: “It’s all about being an educator. We do that in the dorm at night, and I do it on the faculty senate [she is vice chair]. It’s all about how we make this the best educational institution it can be and how we foster the lives of the students who are here. That’s how I’ve gotten engaged in all these things, and it’s been a wonderful experience for me.”
Julie Parsonnet is indeed a most enthusiastic citizen of the university.
Doctors Jason Andrews and Nathan Lo push for cost-effective approach for ridding parasitic worm diseases
Written by Ruthann Richter of the School of Medicine's blog, SCOPE
Scientists call for end to devastating worm diseases
Consider the lowly worm. For some, it’s just a garden pest. But for more than a billion people in the developing world, parasitic worms can be a pernicious threat, causing disease, disability and sometimes death.
In a newly published perspective, Stanford researchers and a host of distinguished colleagues urge the World Health Organization to develop sweeping new guidelines to help end parasitic worm diseases, one of the world’s most prevalent health problems. They call for greatly expanded treatment for these diseases, which could save years of human suffering and an estimated $3 billion in lost productivity — similar to the impact of the Ebola and Zika epidemics of recent years, they say.
“Now everyone is coming together to say, ‘Now is the time, after more than a decade of new experience and data, to update the way we do things,’ Nathan Lo, a Stanford MD/PhD candidate who is the first author, told me. “There is so much opportunity, whether it’s expanding treatment from children to the entire community or bringing in other strategies, such as sanitation, to strengthen the way we approach these diseases.”
Lo’s 17 co-authors include the founders of the field of neglected tropical diseases, as well as leading scientists from the U.S. Agency for International Development, pharmaceutical companies and nonprofits that support treatment programs. Many of them were involved in developing the original strategy for treating parasitic worms in 2001 and now call for change.
The perspective is published today in Lancet Infectious Diseases and coincides with a WHO meeting in Geneva where officials, including many of the authors, are gathering to consider new treatment guidelines.
The worms they are targeting include the Schistosoma parasites and the soil-transmitted helminths, such as hookworm and roundworm, which together afflict an estimated 1.5 billion people in some 112 countries. The Schistosoma worms reproduce in fresh water snails and may infect those who bathe in contaminated rivers and lakes. The soil-transmitted helminths are mainly found in soil, and once they find their way into the body, they produce eggs that can be transmitted to others through skin penetration or ingestion of human feces in soil or water supplies. These parasites can cause symptoms ranging from anemia, malnutrition and growth stunting to infertility, bowel obstruction and fatal liver, bladder and intestinal disease. About 20,000 people die of complications from these parasitic infections every year.
“The World Health Organization has been leading the charge against these infections through its guidance around control strategies,” said Jason Andrews, MD, assistant professor of medicine and senior author of the perspective. “The evidence has evolved over the past decade but the guidelines have not kept up. We found that engaging many of the leading scientists, donors and implementers that there was broad consensus about the need to update the global strategy for schistosomiasis and soil-transmitted helminth control and elimination.”
Current WHO policies, which date back to 2001, call for treating only pre-school or school-aged youngsters with very low-cost medications that effectively knock down the worms. But often the treated children become reinfected by adults or others in their communities.
The scientists argue for expanding drug treatment to entire communities that are at risk, a strategy that has been shown to be highly cost-effective. These mass treatment programs should be combined with other initiatives to improve water quality, sanitation and hygiene practices, as well as programs to control the proliferation of water-borne snails that harbor the worms, they say. The strategies may vary from country to country, depending on needs and resources, and guidance should be given accordingly, Andrews said.
“We have proposed more specific guidance for countries that are seeking to reduce morbidity, versus those that are approaching elimination, as we believe that different strategies are required for different scenarios,” he said.
Previously: To control schistosomiasis, Stanford researchers advise thinking beyond pills, Massive campaign against parasitic worm disease is cost-effective, new study shows and A new framework for expanding treatment guidelines for parasitic worm diseases
Schistosomiasis image by Yale Rosen
Gernot of Bollyky lab awarded DoM's Employee of the Month
Copied from Department of Medicine News
Gernot Kaber says, “Whatever machine, animal model or resource is needed for a project, it can be found somewhere [at Stanford]!” As a basic life research scientist in the Division of Infectious Diseases, Gernot would know. Via what Gernot calls “broad experience” with many different research methods and models, he helps graduate students (and sometimes post docs) with experimental planning, setup and analysis, which ensures that the experiments produce reliable high quality data.
He’s so good at it, Bollyky Lab Manager Heather Ishak says, “Gernot used to be our secret weapon.” However, she adds, “Word got out somehow. Post docs and PIs regularly seek him out from other labs for advice. His positive interactions with other labs are contributing to better science and collaborations here at Stanford.”
The Science
Paul Bollyky also recognizes Gernot’s contributions to science at Stanford. He says, “Dr. Kaber is doing exciting, ground breaking work on a number of projects. His work on molecular biology, animal models of immunologic diseases and histology are critical to the functioning of the lab. He has made himself indispensable.”
Before coming to Stanford to work in the Bollyky Lab in December 2014, Gernot studied pharmacy at the Heinrich-Heine University in Dusseldorf, earned his PhD at the Institute for Pharmacology and Clinical Pharmacology there and then completed a research fellowship at the Benaroya Research Institute in Seattle. While in Dusseldorf, he says, “Most of my work was cardiovascular in nature and done in mice, rats and rabbits. I acquired a lot of experience in small animal work and surgery. My work [in Seattle] was focused on extracellular matrix components and their impact in lung disease and autoimmunity.”
As a result of his expertise and dedication, Bollyky says, “Gernot is a valued contributor to nearly every project going on in the lab and a co-author on three publications in the past year and will be included on several others that are in preparation or in review.”
Putting Others First
This devotion to the work of others can come at a price. “Spending a lot of time to ensure that other people do well with their research and that the lab is productive” makes it challenging, says Gernot, “to keep my own research projects on track.”
Lab manager Heather Ishak sees exactly what Gernot is talking about. She says that he is so dedicated to his “lab family” that “his own productivity should suffer because he is constantly supporting the other research staff, but he makes up for it by working longer and coming in on the weekends to finish projects. He goes above and beyond expectations.”
The practice of putting others’ work ahead of his own is one reason Gernot says the Employee of the Month award feels “great! It is always nice to know that all the hard work is appreciated. It is good to know that others recognize that my input is valuable and helpful.”
And they certainly do. Paul Bollyky says, “Gernot is the hardest working member of the lab. He regularly puts in long hours and is clearly devoted to the success of the projects he is working on and to the lab in general. Gernot is a particularly effective teacher and mentor to junior members of the lab.”
Post-doc Ben Danielson, who has been mentored by Gernot, offers additional details. He calls Gernot a “fantastic scientist” and says, “Gernot is a tireless worker that probably spends more time everyday helping other people with their research than he does on his own projects. Whenever I am having problems with my research (which is quite often), Gernot never hesitates to lend a helping hand and troubleshoot possible solutions. Even on weekend afternoons, he will give up time out of his day to help out and ensure the success of everyone in the lab.”
The “Go-To” Guy
These qualities make Gernot “the ‘go-to’ guy,” says senior staff researcher Nadine Nagy. “He has exceptional scientific knowledge and is a great mentor, which is why everybody – from grad students to PIs – reaches out to him. He is very passionate about science and loves sharing what he knows. He is a fountain of knowledge everyone can count on. He spends multiple hours each week helping others with their experiments, by discussing, troubleshooting and generating new ideas. All in all, he is known for his intelligence and his devotion to work.”
Like Nagy, Ishak sees how much everyone relies on Gernot. She says, “I’ve worked closely with Gernot for two years. He treats the lab as an extension of his own family. Gernot is the ‘go-to’ person for trouble-shooting experiments because not only does he have the expertise to supply practical and technical assistance, but he also makes time to listen and help.”
Post-doc Koshika Yadava agrees and says, “Gernot is the powerhouse of the lab. He is an encyclopedia of scientific methods and is always up for a heated debate on science. As a post doc I have benefited a lot from his knowledge, to design my own research. Besides enriching the lab intellectually, he is also extremely helpful, taking care of the daily mundane tasks so that the lab can function efficiently.”
Gernot is “exactly the kind of scientist every lab manager wants,” concludes Ishak. “He does so much behind the scenes work such as restocking supplies, cleaning the centrifuges, taking out recycling and putting away supplies. If there is a freezer alarming down the hall, he will go report it to the lab. If someone drops a stack of papers in the hall, he helps pick them up. All of these actions are little things that add up to a lot and make for a great working environment.”
Congratulations Dr. Dora Ho on exemplary patient care
Dr. Dora Ho sets the example in patient care at Stanford
A number of faculty members, students, trainees, staff and affiliated staff won awards for dedication to and excellence in graduate and medical education, patient care and teaching.
Dr. Dora Ho has been recognized for her exemplary clinical and humanistic skills by being awarded the Alwin C. Rambar-James B.D. Mark Award for Excellence in Patient Care.
The annual award recognizes a Stanford physician for compassion in working with patients and their families, excellence in providing medical treatment and effectiveness and pleasantness in interactions with patient-care staff. The Rambar award was established in 1985 to honor the late Alwin Rambar, a Chicago pediatrician long associated with the medical school. It was renamed in 1997 to include James Mark, a former chief of staff, Stanford thoracic surgeon and professor emeritus who was also Rambar’s son-in-law.
Paul Bollyky’s Catalyst Award Aims
Squarely at Pseudomonas Infections
Dr. Paul Bollyky, assistant professor of Infectious Diseases and Geographic Medicine
Source: Department of Medicine News
Recently, Paul Bollyky, MD, DPhil (assistant professor, Infectious Diseases), learned that he was to receive a Catalyst Award from the Dr. Ralph and Marian Falk Medical Research Trust. The one-year grant of almost one-half million dollars aims to fund preliminary steps (such as planning, project and team development, purchase of tools, hiring of personnel) that will, according to the Trust, enable awardees to undertake a high risk/high reward project “that addresses critical scientific and therapeutic roadblocks.”
Bollyky’s project aims to solve the problem of antibiotic resistance by the bacteriaPseudomonas aeruginosa, a major cause of pneumonia, infected wounds, and hospital-acquired infections. Antibiotic-resistant infections are not a new phenomenon, but they are a frustration to infectious diseases physicians whose patients have to fight them with fewer options all the time. “These Pseudomonas infections have evolved resistance to most, and in some cases, all of the antibiotics that we have to use against them,” explains Bollyky.
“As an infectious diseases doctor on the wards, I often see patients with these infections. And I have had patients where we’ve amputated limbs because we don’t have any antibiotics that work against their infection. If antibiotic choices are like bullets in a gun, I often find that I’m out of bullets to use against Pseudomonas infections. If we’re lucky we may come up with another solution for treating these bugs.”
Bollyky’s target is a bacteriophage, or a virus, that infects bacteria like Pseudomonas. While most bacteriophages parasitize bacteria, Bollyky and his collaborators discovered that certain bacteriophages actually partner with their bacterial hosts. In a recent article in Cell Host & Microbe (2015;18:549-59), they showed that these bacteriophages play a pivotal role in the formation of biofilm, which is an adherent coating that allows bacteria to colonize surfaces like wounds and catheters. Both bacteriophage and bacteria thereby work together to cause infections. “What that means,” Bollyky explains, “is that we have a target that we can go after to treat antibiotic-resistant Pseudomonas infections.”
He continues: “I think the reason why the Falk Trust gave us this funding is because we have this novel target that puts another bullet in the gun, so to speak. In particular, the Catalyst Award is funding a pair of strategies to develop therapies that target this family of bacteriophages, and we’re optimistic that one and maybe both strategies will give us some novel therapies to treat multi-drug resistant Pseudomonas.”
In order to jump-start this project, Bollyky plans to use the Catalyst Award monies to hire a dedicated microbiologist, add needed equipment in his lab, and invest in reagents and materials to develop his technology. “What I’m most excited about,” he says, “is getting a slug of resources and, to be honest, a vote of confidence from a handful of reviewers. This gives us both the momentum and the resources to take this to the next level. It propels the whole lab in a new and pretty exciting direction.”
This breadth of science has allowed him to rapidly integrate and contribute greatly to the Stanford academic/intellectual environment
Ideally, a successful, milestone-driven Catalyst Award will lead to another grant, a two-year Transformational Award from the Falk Trust, which offers $1 million of support to approximately half of Catalyst awardees following a second round of competitive applications.
Upinder Singh, MD (chief, Infectious Diseases and Geographic Medicine) cites two features that make Dr. Bollyky such a successful scientist: “One is his ability to think outside the box, and the second is the overall breadth of his research program (ranging from autoimmunity, inflammation, to antibiotic resistance [this grant] to biofilm formation). This breadth of science has allowed him to rapidly integrate and contribute greatly to the Stanford academic/intellectual environment. He has already collaborated (and in some cases published) with a number of faculty (including in Bone Marrow Transplant, Neurology, Immunology, Pediatrics).
Asked how he feels about having received this first award from the Falk Trust, Bollyky says: “To use an outer-space metaphor, the Catalyst Award is the first stage that allows this project — and optimistically my lab — to get off the ground. And then if we make the second stage of the Falk funding, the Transformational Award, then that’s the booster that will really put us into orbit.”
We hope to report on the orbiting Bollyky lab in about a year.
Stanford Researchers Uncover New Bacterial Diversity Inside U.S. Navy Dolphins
- Steve Fyffe, from Stanford CISAC

Chairman of Joint Chiefs of Staff Gen. Martin E. Dempsey watches a dolphin jump out of the water during a tour of the U.S. Navy Marine Mammal Program in San Diego on March 12, 2012.
Photo credit:
U.S. Navy
Stanford researchers working with the U.S. Navy’s Marine Mammal Program in San Diego have discovered a startling variety of newly-recognized bacteria living inside the highly trained dolphins that the Navy uses to protect its ships and submarines, find submerged sea mines and detect underwater intruders. They found similar types of bacteria in wild dolphins as well.
“About three quarters of the bacterial species we found in the dolphins’ mouths are completely new to us,” said David Relman, Stanford professor of microbiology and medicine, and co-author of a paper published in the journal Nature Communications on Wednesday.
 These previously unknown bacteria represent “whole new realms of life,” according to Relman.
These previously unknown bacteria represent “whole new realms of life,” according to Relman.
“Bacteria are among the most well-studied microbes, so it was surprising to discover the degree to which the kinds of bacteria we found were types that have never been described,” he said. “What novelty means is not just new names of species, families, classes or phyla…there’s reason to believe that along with this taxonomic novelty, there’s functional novelty.”
The U.S. Navy has been training dolphins and sea lions to carry out defensive military missions from their bases in San Diego and elsewhere since the early 1960s.
Over the years, it has also funded scientific research and become the single largest contributor to the scientific literature on marine mammals, producing more than 800 publications, according to the Navy.
Relman started working with the Navy more than 15 years ago to identify bacteria suspected of causing stomach ulcers in their dolphins.
His latest project to catalog the bacterial communities (or microbiota) living inside the dolphins began when the Navy asked him to help develop a probiotic bacterial strain that could keep their dolphins healthy, or help sick dolphins get better.
Navy trainers took regular swabs from the dolphins’ mouths and rectal areas, using what looked like a Q-tip, and shipped the samples to Stanford on dry ice for analysis.
 They also collected samples of the air the dolphins exhaled from their blowholes (known as “chuff”) onto sterile filter paper, as well as samples of their gastric juices using a tube that the dolphins would swallow on command, and for comparison, bacteria from the surrounding water.
They also collected samples of the air the dolphins exhaled from their blowholes (known as “chuff”) onto sterile filter paper, as well as samples of their gastric juices using a tube that the dolphins would swallow on command, and for comparison, bacteria from the surrounding water.
The study found a similar amount of diversity and novelty in bacterial samples taken from wild dolphins living in Sarasota Bay off the west coast of Florida, although there were slight differences in the bacteria from the dolphins’ mouths.
Relman said he hoped to develop a profile of the normal microbial communities in healthy dolphins and other marine mammals, so that scientists could detect any early change that might signify an imminent disease, or health problems caused by climate change and ocean warming.
“There’s a lot of concern about the changing conditions of the oceans and what the impact could be on the health of wild marine mammals,” Relman said. “We would love to be able to develop a diagnostic test that would tell us when marine mammals are beginning to suffer from the ill effects of a change in their environment.”
The research could help solve other mysteries, such as how dolphins digest their food, even though they swallow fish whole without chewing them.
The key could be a unique bacterial group that’s also been identified in an endangered species of freshwater dolphins living in China’s Yangtze River, said Elisabeth Bik, a research associate at the Stanford Department of Medicine and lead author on the paper.
“It’s a very intriguing bacterial group that nobody has seen before in any other terrestrial animal group,” said Bik. “I would really love to know more about those bacteria and sequence their genomes to understand more about their functional capacity.”
 The study also examined oral, gastric and rectal samples from the Navy’s trained sea lions.
The study also examined oral, gastric and rectal samples from the Navy’s trained sea lions.
“The sea lions and dolphins are kept at the same facility, they’re fed exactly the same fish, and they’re swimming in the same water…but they’re very, very different in terms of microbiota,” Bik said.
Unlike dolphins, sea lions share many common types of bacteria with their terrestrial cousins.
“Sea lions weren’t that different from other carnivores like dogs and cats,” Bik said. “They’re evolutionarily related to them, and their microbiota looks very similar to those animals. But dolphins don’t really have a terrestrial mammal that’s closely related, and their microbiota looks very different from anything else that people have seen.”
Relman said his team was planning on expanding their study to include other marine mammals such as sea otters, killer whales, baleen whales, grey whales, harbor seals, elephant seals and manatees. Their purpose, in part, is to understand how life in the sea, over the millions of years since the return of mammals, may have shaped the structure of their microbial communities and the roles they play in marine mammal health.
They’re already working to analyze more than 80 samples of killer whale stool that the U.S. National Oceanic and Atmospheric Administration has gathered with the help of specially trained sniffer dogs, which stand on the bow of their boats and point to fresh killer whale feces before it sinks.
The California Department of Fish and Wildlife is contributing samples from the sea otters and seals it studies as part of its conservation, ecological, and monitoring programs.
And the Marine Mammal Center in Sausalito, which is the West Coast’s largest rescue and rehabilitation facility for marine mammals, is sending samples from the seals in its care.
Relman said the research could help scientists begin to answer fundamental questions about life in the ocean.
“Marine mammals remain one of the more poorly understood habitats for studying microbial life, and there would be lots of reasons for thinking that these are important environments to study, in part because of the relevance for the health of these marine mammals, but also because they represent a view into what it means to live in the sea and the nature of our relationship with this vast aspect of our environment,” Relman said.
Collaborators and co-authors on this study included Stephanie Venn-Watson and Kevin Carlin from the National Marine Mammal Foundation, and Eric Jensen from the Space and Naval Warfare Systems Center Pacific, in San Diego.
Senior Author Dr. Catherine Blish researches link HIV susceptibility to little-understood immune cell class
The white cells are responsible for attacking viruses and tumors
Juan Gaertner/Shutterstock
By Bruce Goldman, Department of Medicine News
High diversity among certain cells that help fight viruses and tumors is strongly associated with the likelihood of subsequent infection by HIV, Stanford University School of Medicine researchers have found.
Natural killer cells, or NK cells, are lymphocytes, a type of white blood cell. NK cells’ increased diversity, the scientists learned, may stem from prior exposures to viruses.
The findings, described in a study published July 22 in Science Translational Medicine, could spur the development of blood tests capable of flagging individuals’ susceptibility to viral infection. The study also offers insights into the workings of NK cells, a somewhat poorly understood but crucial group of immune cells.
“This puts NK-cell diversity on the map as a metric of immune function,” said Catherine Blish, MD, PhD, assistant professor of infectious diseases and geographical medicine and the study’s senior author. “But it was a first foray. Before we can say definitively that NK-cell diversity predicts a person’s susceptibility to infection, we need to validate these findings by looking at large numbers of individuals in a different population.
“NK cells are particularly suited to detecting and demolishing virally infected or cancerous cells,” Blish added. “They arrive on the scene quickly, and they act quickly. An NK cell can kill an infected cell in 10 minutes.”
Unexpected finding
Unexpectedly, it was higher, rather than lower, diversity in this immune-cell population that turned out to be associated with increased HIV susceptibility in the study. The investigators had figured that, as is the case with B cells and T cells — the two other, better-known types of lymphocytes — diversity in NK cells would be a strength, not a detriment, Blish said.
“Our hypothesis was wrong,” she said. “We didn’t think NK-cell diversity would be a bad thing, or that NK cells’ diversification would occur to the extent that it does with viral exposure.”
Using a cutting-edge, single-cell analytic technology called mass cytometry, Blish and her colleagues, including the study’s lead author, graduate student Dara Strauss-Albee, showed that overall diversity in people’s NK-cell repertoires is low at birth and steadily accumulates over the course of a lifetime.
An individual T cell has surface receptors that recognize unique protein snippets, called peptides, on other cells’ surfaces. The structures of these receptors, which can discern “healthy” versus “suspect” peptides, differs from T cell to T cell. So a healthy person’s legion of T cells can surveil and sort out hundreds of millions of different peptides representing possible invaders.
Unlike their T cell cousins, NK cells don’t have surface receptors that recognize unique peptides. Instead, these lymphocytes harbor various combinations of generic receptors. Some receptors recognize signs of other cells’ normalcy, and others recognize signs that a cell is stressed — due, say, to viral infection or cancerous mutation. On recognizing their targeted features on other cells’ surfaces, an NK cell’s “normalcy” receptors tend to inhibit it, while its stress-recognizing receptors activate it.
All told, NK cells can have many thousands of different combinations of these receptors on their surfaces, with each combination yielding a slightly different overall activation threshold. An NK cell’s surface features also vary depending on its degree of maturation.
Mass cytometry analysis of NK cells exposed in a dish to HIV — as well as to West Nile virus, which differs substantially from HIV in its makeup and its modus operandi — showed that exposure to virus-infected cells leads to differentiation of NK cells and to an increased diversity among them. But diversification in the NK-cell population, the experiments indicated, was associated with a diminished ability of these cells’ ability to replicate and kill.
The researchers also showed that while healthy human adults differ considerably from one another in the diversity of their NK-cell populations, a given adult’s NK-cell population remains quite stable, changing little over periods of many months. An examination of NK cells extracted from umbilical-cord blood showed that newborns’ NK-cell population is much less diverse.
Blish said she believes that viral exposure during one’s lifetime is the driving force behind the maturation, differentiation and diversification of NK cells.
Blish said she believes that viral exposure during one’s lifetime is the driving force behind the maturation, differentiation and diversification of NK cells.
HIV link
In order to assess the impact of NK-cell diversity on adult humans’ viral susceptibility, Blish and her associates turned to blood samples that had been drawn during the Mama Salama Study, a longitudinal study of just over 1,300 healthy pregnant or postpartum Kenyan women. In that study, 25 of the women were found to have HIV. For 13 of these women, blood drawn both before and after infection was available.
This puts NK-cell diversity on the map as a metric of immune function.
Using mass cytometry, the researchers carried out a precise analysis of NK cells in the women’s blood and observed a strong positive correlation between the diversity of a woman’s NK cell population and her likelihood of becoming infected with HIV. This correlation held up when the scientists controlled for age, marital status, knowledge of sexual partners’ HIV status and history of trading sex for money or goods. The two groups of women were also statistically indistinguishable with respect to their sexually transmitted disease status or their reported frequency of recent unprotected sex.
The NK-diversity-dependent difference in these women’s likelihood of HIV infection was huge. Those with the most NK-cell diversity were 10 times as likely as those with the least diversity to become infected.
A 10-fold risk increase based solely on NK-cell diversity is far from negligible, said Blish. “By way of comparison, having syphilis increases the risk of contracting HIV two- to four-fold, while circumcised men’s HIV risk is reduced by a factor of 2.5 or 3,” she said.
The observations could have clinical potential, most immediately by spotlighting people who need to be closely monitored for possible viral infections and, perhaps, prophylactically treated. But Blish cautioned that the study remains preliminary.
Other Stanford co-authors are professor of statistics Susan Holmes, PhD; statistics graduate student Julia Fukuyama; research assistant Emily Liang (now a medical student at UCLA); and immunology graduate student Justin Jarrell.
The study was funded by a Beckman Young Investigator Award, a National Institutes of Health New Innovator Award (grant DP2AI11219301) and a National Science Foundation training grant.
Zika infection causes developing cranial cells to secrete neurotoxic levels of immune molecules
New research shows that cranial neural crest cells can be infected by the Zika virus, causing them to secrete high levels of cytokines that can affect neurons in the developing brain.
SEP 29 2016
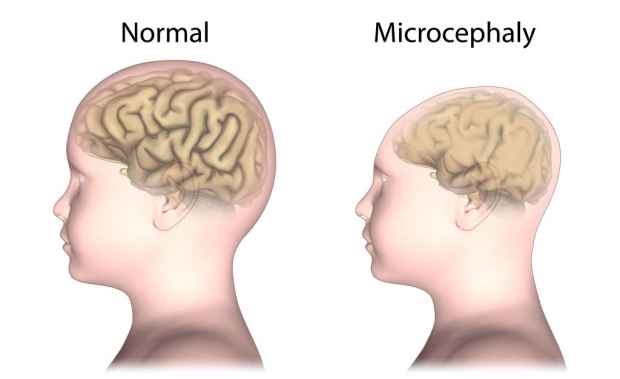
Babies born to women infected with the Zika virus can suffer from a birth defect called microcephaly, or abnormally small heads. A new study from Stanford found that the infection can affect the cells that give rise to the bones and cartilage of the skull and face.
Alila Medical Media/Shutterstock
Infection by the Zika virus causes a population of cells in the cranium of a developing embryo to secrete neurotoxic levels of immune signaling molecules called cytokines, according to a new study by researchers at the Stanford University School of Medicine.
Although the study was conducted solely in cells grown in a laboratory dish, it may provide one possible explanation for why babies born to women infected with the virus can suffer from a birth defect called microcephaly, or abnormally small heads.
“Affected babies have small brains and small skulls,” said assistant professor of medicine Catherine Blish, MD, PhD. “Cells in the cranial neural crest give rise to the bones and cartilage of the skull and face, and they also form an important supportive niche for the developing brain. We wondered if the Zika virus could infect cranial neural crest cells, perhaps giving rise to deficits in skull formation and altered neural development.”
The study was published online Sept. 29 in Cell Host & Microbe. Blish is the senior author. Graduate students Nicholas Bayless and Rachel Greenberg share lead authorship of the study.
A reservoir for the virus
Cranial neural crest cells are cells that arise in humans within about five to six weeks of conception. Although they first appear along what eventually becomes the spinal cord, the neural crest cells migrate over time to affect facial morphology and differentiate into bone, cartilage and connective tissue of the head and face. They also provide critical molecular signals that support nearby developing neurons in the brain.

Catherine Blish
Bayless and Greenberg used a technique developed in the laboratory of study co-author Joanna Wysocka, PhD, a professor of chemical and systems biology and of developmental biology, to convert human embryonic stem cells into cranial neural crest precursors in the laboratory. Wysocka’s research focuses on understanding how these cells affect the embryonic development of facial features, including those of humans and chimpanzees.
Recent research has focused on the effect of Zika virus infection of the neural precursor cells that give rise to neurons in the developing brain. But Bayless and Greenberg found that not only can cranial neural crest cells also be infected by the Zika virus, they respond differently than their neighboring neural precursor cells to the infection. Rather than rapidly dying, as the neural precursors do, the cranial neural crest cells act as a reservoir for the virus by allowing it to replicate repeatedly. In addition, they begin to secrete high levels of cytokines, including leukemia inhibitory factor and vascular endothelial growth factor, known to affect neural development.
“The magnitude of altered cytokine secretion caught us by surprise,” said Blish. “These molecules are important for neurogenesis, and the infected cells are secreting them at high levels.”
Abnormally shaped cells
When Bayless and Greenberg incubated neural precursor cells together with infected neural crest cells, the neural precursors appeared abnormal and were more likely to initiate a program of cellular suicide.

Joanna Wysocka
They next exposed the neural precursor cells to leukemia inhibitory factor and vascular endothelial growth factor at levels equivalent to those secreted by the infected cells. After three days, they observed an increase in structures associated with cellular migration and growth, as well as a significant increase in the frequency of cell death.
“At least in vitro, these elevated levels of cytokines appear to induce premature differentiation and migration,” said Wysocka. “This abnormal developmental program then leads to cell death.”
The Zika virus, which is spread by the Aedes genus of mosquito, has sprung into the public eye over the past year as it has become increasingly apparent that pregnant women infected with the virus can pass it to the fetus, causing devastating birth defects. Outbreaks of the virus are currently occurring in multiple countries and in three American territories: American Samoa, Puerto Rico and the U.S. Virgin Islands. Local mosquito-borne transmission has also been identified in two areas of Miami, and the World Health Organization has designated the disease a public health emergency of international significance.
“Our study brings attention to the possibility that other infected embryonic cell types in the developing head can influence Zika-associated birth defects, including microcephaly, perhaps through signaling to neighboring cells or by serving as a viral replication reservoir,” said Wysocka. “Formation of the cranial neural crest cells and a cross-talk between brain and craniofacial development occurs during the first three months of human fetal development, which is when epidemiological studies have suggested that Zika infection correlates with poor birth outcomes.”
The researchers said that although the findings are intriguing and merit further study, their studies were conducted only on cells grown in a laboratory and it is possible that other factors related to Zika infection may affect brain size and outcomes in affected infants.
Tomek Swigut, PhD, a senior scientist in Wysocka’s lab, is also a co-author of the study.
The research was supported by the National Institutes of Health (grants 1DP2AI112193 and DE024430), the Tashia and John Morgridge Faculty Scholar Program from the Stanford Child Health Research Institute, the March of Dimes Birth Defect Foundation, the Howard Hughes Medical Institute, a seed grant from the Stanford Center for Systems Biology and two Ruth L. Kirschstein awards.
Stanford’s Department of Medicine also supported the work.
- PRESS RELEASES

By KRISTA CONGER
Krista Conger is a science writer for the medical school's Office of Communication & Public Affairs. Email her at kristac@stanford.edu.