Products for In-Vitro Use:
Stanford Blood Center provides blood products for in-vitro investigational use. Tubes or whole units of blood may be collected from individuals selected by the researcher or recruited by the Blood Center. Products collected for in-vitro investigational use are not routinely tested for infectious disease markers, although donor pre-screening can be arranged. Because products for in-vitro use do not meet the same safety criteria as blood products for transfusion, these components must not be used for infusion or for manufacturing into infusible products. Please contact Customer Relations at 650-724-2997 for more information.
Products for In-Vivo Use (Clinical Trials):
Stanford Blood Center collects blood products for investigational in-vivo use. Products may be collected from patients for re-infusion into the same patient (autologous donors), from relatives of patients (directed donors) or from normal healthy donors (allogeneic donors). IRB approval is required for all trials involving investigational in-vivo use of blood products. For information about our clinical trials program, please contact Customer Relations at 650-724-2997.
Product Specifications Legend
| HCT | Hematocrit |
| Lymph | Lymphocytes |
| Mono | Monocytes, eosinophils, basophils, and other large mononuclear cells such as blasts, metamyelocytes, myelocytes, etc. |
| Gran | Granulocytes, including Neutrophils, Eosinophils and Basophils |
The following products are available for in-vivo or in-vitro use (except as noted):
Leukocytes (White Blood Cells)
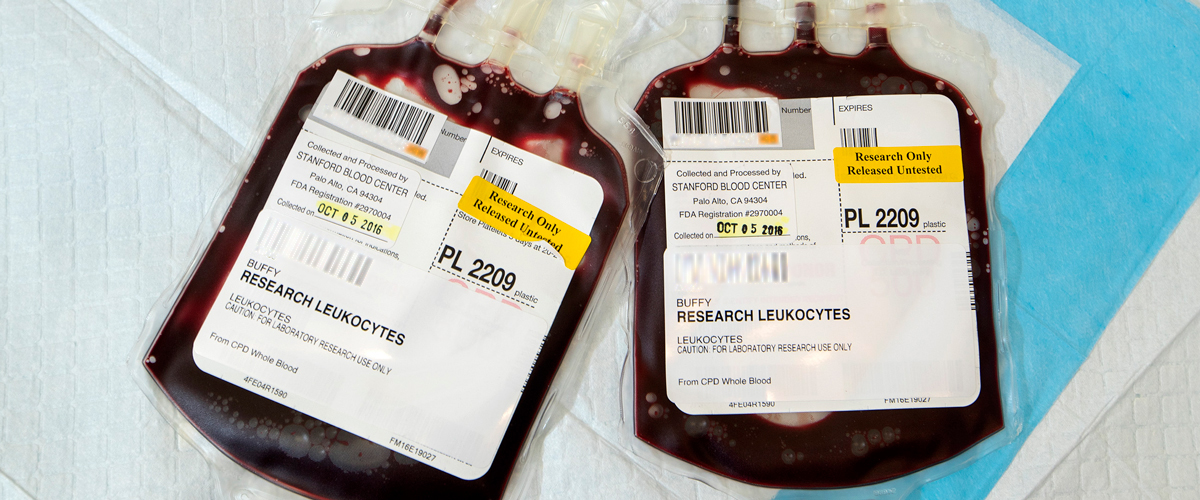
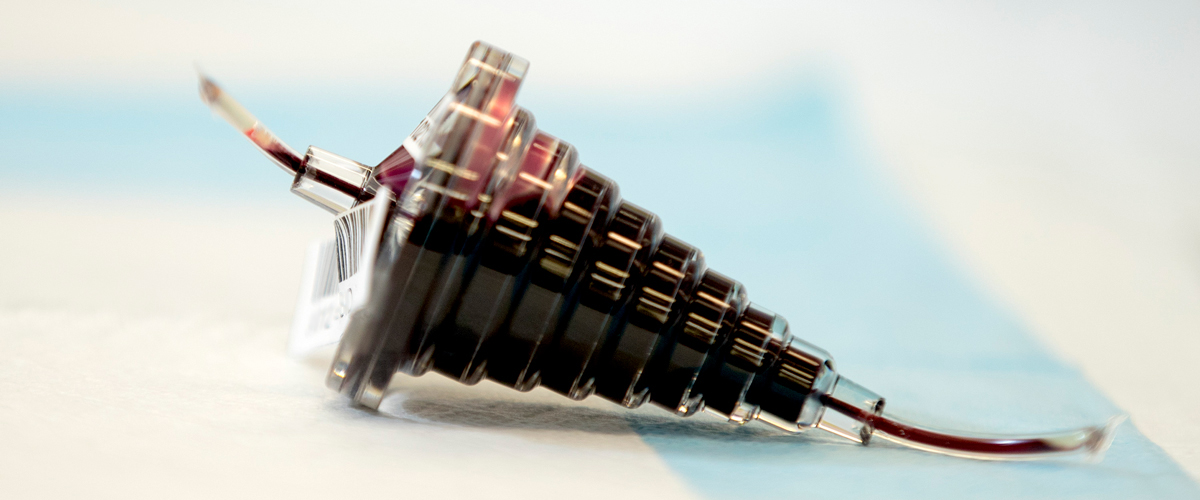
White blood cell concentrate prepared from a single unit of whole blood by centrifugation. Product contains red blood cells and plasma, and negligible amount of anticoagulant (CPD or CPDA-1). Store at 20-24C up to 24 hours.
|
Mean
|
Range
|
||
|
Min.
|
Max
|
||
| Vol (mL) |
30.5
|
23
|
38
|
| WBC thsn/mm3 |
49.8
|
21
|
94
|
| WBC (e9) |
1.5
|
0.6
|
2.7
|
| HCT % |
36.8
|
15.2
|
65.7
|
| % Gran |
47.7
|
28.7
|
67.7
|
| % Lymph |
35.6
|
21.7
|
59.1
|
| % Mono |
10.4
|
4.9
|
18.7
|
White blood cell concentrate of TrimaAccel® LRS chamber recovered after Plateletpheresis procedure. Product contains red blood cells, plasma and negligible amount of anticoagulant (ACD-A). Store at 20-24C up to 24 hours.
|
Mean
|
Range
|
||
|
Min.
|
Max
|
||
| Vol (mL) |
7.5
|
7
|
8
|
| WBC thsn/mm3 |
128.4
|
62.4
|
285.6
|
| WBC (e9) |
1.0
|
0.5
|
2.2
|
| HCT % |
2.3
|
1.9
|
2.5
|
| % Gran |
3
|
0.9
|
8.7
|
| % Lymph |
76.7
|
60.7
|
87.7
|
| % Mono |
19.7
|
9.1
|
32.4
|
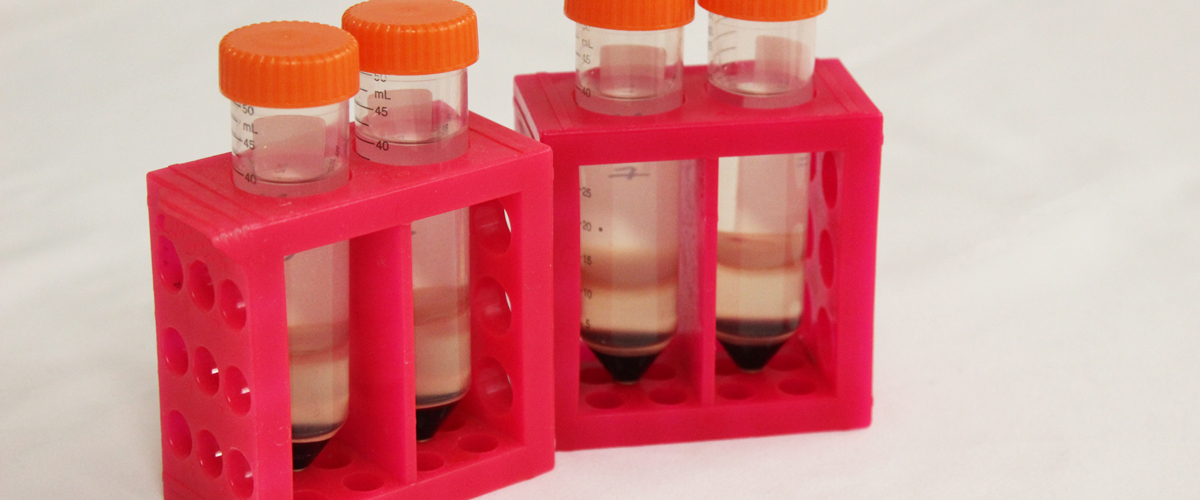
Mononuclear cells collected from a single donor by a centrifugation apheresis technique. Cells are suspended in plasma with ACD-A anticoagulant added. Product is stored at room temperature (20 – 24C) for up to 24 hours.
| Median | Range | ||
| minimum | maximum | ||
| WBC thsn/mm3 | 77.6 | 50.4 | 127.7 |
| HCT % | 5.8 | 2.2 | 11.8 |
| Vol (mL) | 132 | 100 | 184 |
| Total WBC (e9) | 10.2 | 5.0 | 23.5 |
| % Lymph | 73.2 | 37.4 | 78.9 |
| % Mono | 15.2 | 12.2 | 20.2 |
| % Gran | 12.3 | 8.5 | 46.5 |
| Lymph thsn/mm3 | 53.0 | 36.7 | 69.0 |
| Total Lymph (e9) | 7.0 | 3.7 | 12.7 |
| Mono thsn/mm3 | 11.3 | 6.5 | 25.8 |
| Total Mono (e9) | 1.5 | 0.7 | 4.7 |
| Gran thsn/mm3 | 7.7 | 5.2 | 53.7 |
| Total Gran (e9) | 1.0 | 0.5 | 9.9 |
Please contact us for current availability and more information.
Plasma
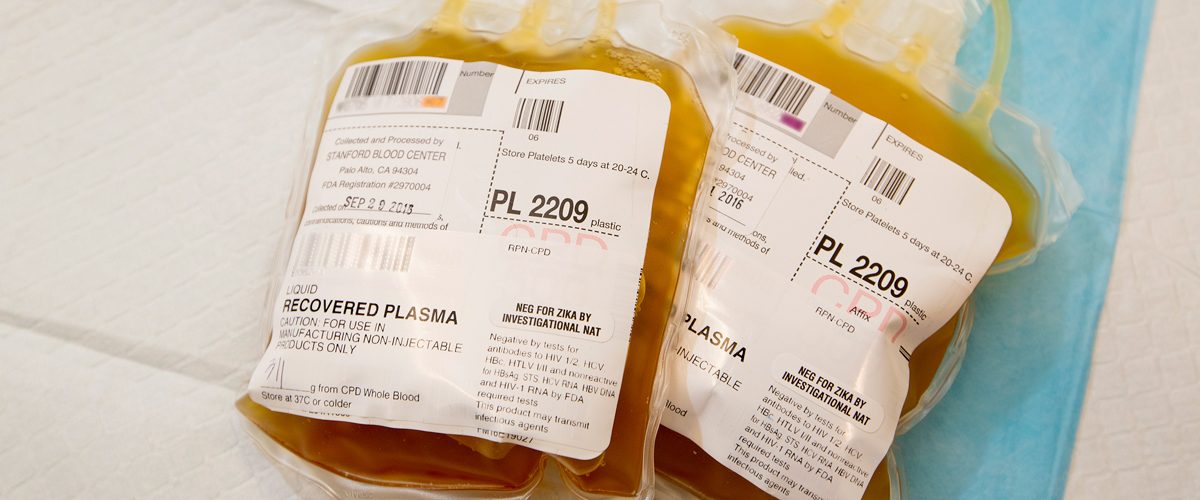
Plasma separated from whole blood by centrifugation (200 mL product, anticoagulant CPD or CPDA-1) or collected by a centrifugation apheresis technique (400 mL product, anticoagulant ACD-A). Frozen at -18C within 8 hours of collection (6 hours for ACD-A plasma), FFP contains plasma proteins including all coagulation factors.
| Volume | ~ 200 or 400 mL |
| Fibrinogen | 2-4 mg/mL |
| Coagulation Factors | 1 IU/mL |
| Storage, frozen | < -18C, 12 months |
| Storage, thawed | 1-6C, 24 hours |
Plasma separated from whole blood by centrifugation and frozen at -18C between 8 and 24 hours of whole blood collection. Contains Factor VIII:C as well as other labile and stable coagulation factors, anticoagulant CPD or CPDA-1.
| Volume | ~200 mL |
| Factor VIII:C | ~150 IU |
| Storage, frozen | < -18C, 12 months |
| Storage, thawed | 1-6C, 24 hours |
Fluid portion of whole blood separated by centrifugation from whole blood unit (200 mL product, anticoagulant CPD or CPDA-1) or by apheresis (400 mL product, anticoagulant ACD-A). Contains plasma proteins including stable coagulation factors.
| Volume | 200 or 400 mL |
| Fibrinogen | ~2-4 mg/mL |
| Stable coagulation factors only* | 1 IU/mL |
| Storage | 1-6C, 24 hours |
Plasma remaining after removal of cryoprecipitated AHF. Significantly reduced clotting factors and fibrinogen.
Platelets
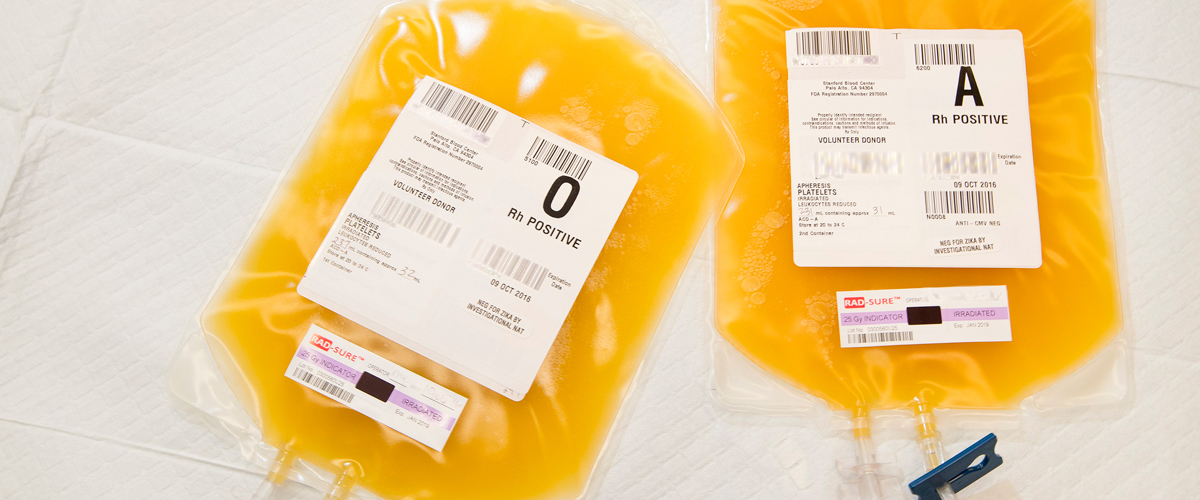
Platelets collected from a single donor by a centrifugation apheresis technique. Each product contains platelets suspended in plasma and anticoagulant ACD-A.
| Volume | 200-300 mL |
| Platelets | > 3.0 x 10 11 |
| Storage | 20-24C, continuous gentle agitation, 5 days |
Plasma up to 200 mL collected from a single donor concurrently with an apheresis platelet collection. See Fresh Frozen Plasma and Plasma, liquid.
Unless specified, platelets, pheresis undergo white blood cell reduction during the automated collection process, resulting in residual WBC < 5.0 x 10 6.
Red Blood Cells
Additive red blood cells may be prepared by centrifuging whole blood to remove as much plasma as possible, and replacing the plasma with Adsol (see Legend ). Cells have lower viscosity than CPD or CPDA-1 Red blood Cells, and flow in a manner more comparable to that of whole blood.
AS-1 (WB)
| Volume (RBC+Plasma) | ~300 mL |
| HCT | 55-65% |
| Storage | 1 – 6C, 42 days |
AS-1 (Aph Double Red Cells)
| Volume | ~413-490 mL |
| Absolute RBC volume | 360-420 mL |
| Storage | 1 – 6C, 42 days |
Prepared from whole blood collected into CPD or CPDA-1 anticoagulant-preservative (see Legend ), and separated from the plasma by centrifugation or sedimentation.
| Volume | ~230 mL |
| HCT | 65-80% |
| Storage, CPD | 1 – 6C, 21 days |
| Storage, CPDA-1 | 1-6C, 35 days |
Prepared by filtering red blood cells with Fenwal CPDA-1 or Fenwal CPD/ADSOL Leukocyte Filters. Filtering may be done within 3 days of draw. Residual leukocytes in filtered red blood cells are <5 x 10 6 with retention of 85% of the original component.
Red blood cells, or leukoreduced red blood cells derived from 475 mL of whole blood, further centrifuged to achieve final HCT of >85%. Not available in AS-1 anticoagulant-preservative.
Whole Blood
Whole blood released within 4 hours of collection, untested, or within 24 hours of collection, tested.
| Volume | 500 mL (±10%) |
| HCT | > 34% |
| Storage, CPD | 1 – 6C, 21 days |
| Storage, CPDA-1 | 1-6C, 35 days |
Tubes Only
There is no additive or anticoagulant added to this tube.
Used for specific procedures requiring serum.
Contains 0.07 mL of 15% buffered solution or the calculated equivalent of 10.5 mg EDTA (K 3). EDTA or ethylenediamineteteracetic acid chelates calcium to prevent clotting.
Used primarily for hematology and specific chemistry procedures requiring plasma or whole blood. EDTA whole blood samples are superior for flow cytometry studies because the formation of artifacts is prevented, even after prolonged standing.
Contains 0.050 mL of 15% buffered solution or the calculated equivalent of 7.5 mg EDTA (K 3). EDTA or ethylenediamineteteracetic acid chelates calcium to prevent clotting.
Used primarily for hematology and specific chemistry procedures requiring plasma or whole blood. EDTA whole blood samples are superior for flow cytometry studies because the formation of artifacts is prevented, even after prolonged standing.
Contains 1.50 mL of anticoagulant Acid Citrate Dextrose Solution (A). ACD-A contains nutrients to help maintain the whole blood for several weeks.
Used primarily for specific immunohematology and histocompatibility procedures requiring preservation of whole blood.
Contains 143 USP Units of Sodium Heparin. Heparin inhibits thrombin to prevent clotting.
Used for chemistry and selective hematology determinations. This anticoagulant is best for prevention of hemolysis and for osmotic fragility tests.
Anticoagulants and Additive Solutions
ACD-A Anticoagulant Citrate Dextrose solution USP (ACD) Formula A
- Dextrose (monohydrate)
- Sodium Citrate (dihydrate)
- Citric Acid (anhydrous)
AS-3 Additive Solution Formula 3 (100mL added to ACD-A Red Blood Cells collected by apheresis)
Each 100 mL contains:
- Dextrose (monohydrate), USP 1.10 g
- Sodium Citrate (dihydrate), USP 0.588 g
- Sodium Chloride, USP 0.410 g
- Monobasic Sodium Phosphate (monohydrate), USP 0.276 g
- Citric Acid (monohydrate), USP 0.042 g
- Adenine 0.030 g
CPD Anticoagulant Citrate Phosphate Dextrose USP
- Sodium Citrate (dihydrate) 26.3 g/L
- Dextrose (monohydrate) 25.5 g/L
- Citric Acid (anhydrous) 3.27 g/L
- Monobasic Sodium Phosphate (monohydrate) 2.22 g/L
AS-1 Adsol® additive formula (added to CPD Red Blood Cells)
- Sodium Citrate (dihydrate) 26.3 g/L
- Dextrose (monohydrate) 25.5 g/L
- Citric Acid (anhydrous) 3.27 g/L
- Monobasic Sodium Phosphate (monohydrate) 2.22 g/L
- Sodium Chloride
- Mannitol
- Adenine
CPDA-1 Anticoagulant Citrate Phosphate Dextrose Adenine Solution USP
- Dextrose (monohydrate) 31.9 g/L
- Sodium Citrate (dihydrate) 26.3 g/L
- Citric Acid (anhydrous) 3.27 g/L
- Monobasic Sodium Phosphate (monohydrate) 2.22 g/L
- Adenine 0.275 g/L
(In order of Research Products and Services tables)
