Study Navigator News
Study Navigator 2.0
Study Navigator was redesigned in fall 2011 in response to user feedback and to better integrate it with our redesigned website.
Please update your bookmarks accordingly. Make sure you log in (top right of page) and go to the main Navigator page to access your studies or create a new study.
See below for some screenshots of the new features.
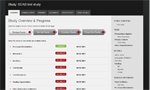
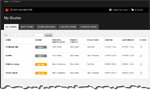
Study Overview
New layout and organization with accordions and tabs with a “service completion” feature.
We are continually looking to improve the user experience with the Study Navigator.
These are the major features introduced in 2012:
- • CTRU services: incorporation of access to services
- • Extended scheduling: combining calendars of multiple providers
- • Synchronization with other administrative systems: OnCore, to ensure proper tracking and minimize duplicate data entry
Major features introduced in 2013:
- • CTRU services: dashboard overview of CTRU requests including sorting by submissions and document updates
- • Unobtrusive warning and security messages for users at the top of the screen
- • Improved document security for PIs and Primary contacts with budget providers
Major features in the works:
- • Synchronization with other administrative systems: SeRA, eProtocol, to ensure proper tracking
- • Community Engagement services: access technical assistance appointments
If you have any specific suggestions, please send an email to clinicaltrials@med.stanford.edu.
Study Navigator

Starting a Clinical Study?
Study Navigator is a study management tool for researchers for developing and conducting clinical trials or human studies at Stanford. Use Study Navigator to schedule consultations, download tools, update studies.
Login to get startedAlerts
PMCID Requirement
Each publication, press release, or other document that cites the results from NIH grant-supported research must be submitted to PubMed Central, and the PMCID number must be reported.
Learn more