A central tenet of biology is that each amino acid in a protein is specified by a three-letter code found in messenger RNA (mRNA). That may be true most of the time, but HHMI Investigator Jonathan Weissman at the University of California, San Francisco (UCSF), along with Onn Brandman at Stanford University and UCSF colleague Adam Frost, has discovered an exception to the rule.
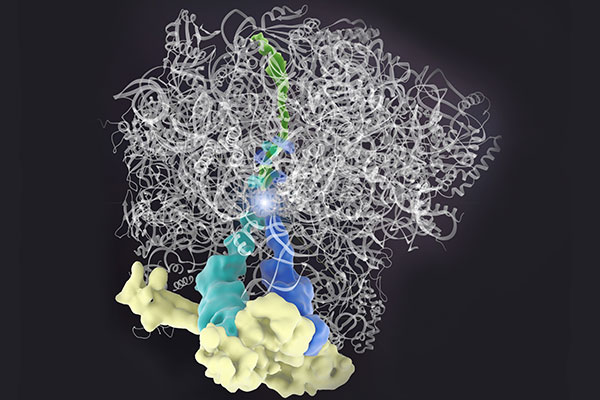
Generally, protein synthesis requires three components: an mRNA template, a ribosome to read the template and assemble the protein, and transfer RNA (tRNA) molecules to supply the necessary amino acids. Weissman and his colleagues discovered that sometimes, when protein synthesis stalls, elongation can still continue without the mRNA template. This type of synthesis relies on only two components: a part of the ribosome called the 60s subunit and a protein named Rqc2 that recruits tRNA molecules. With these components, cells will continue to randomly add amino acids to the stalled peptide, with one caveat. Rqc2 recognizes only alanine and threonine (abbreviated “A” and “T”), so it creates what Weissman refers to as “CAT tails.”
“This is a mode of protein synthesis that we’ve missed for 50 years. It’s totally unprecedented,” says Weissman. He and his colleagues published their findings January 2, 2015, in Science. They don’t yet know the function of CAT tails, but they have some ideas they’re pursuing. For example, the extra amino acids may trigger a stress response in the cell, or they could tag an incomplete peptide for destruction.