 |
|
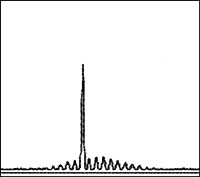
| B-cell clonality assay. DNA from a normal or polyclonal B cell population produces a bell-shaped curve of amplicons (Gaussian distribution) that reflect the heterogeneous population of VDJ region rearrangements. A positive result is evident as presence of a predominant peak. | |
Hematolymphoid Malignancies
Acute Leukemia
Multiple acute leukemia assays are offered. Mutations and internal tandem duplications that produce increased activity of the enzyme coded by the FLT3 gene may be seen in patients with acute myelogenous leukemia (AML) and may be associated with increased risk of relapse, whereas insertion mutations in exon 12 of NPM1 may confer a favorable prognosis. We offer an assay based on fluorescent PCR combined with capillary electrophoresis, restriction enzyme digestion and GeneScan analysis to detect these changes. Another example is a molecular assay for promyelocytic leukemia. In acute promyelocytic leukemias (APL – M3), 98% of patients carry the t(15;17) translocation, which may be identified and monitored in the laboratory by quantitative RT-PCR. These patients must be identified early, since they are at risk of life-threatening coagulopathy and the therapy differs from other acute leukemias. We also offer an assay for CEBPa, which, when two mutations are present, can indicate a favorable prognosis. In addition, we perform testing for KIT.
BCR-ABL for Chronic Myelogenous Leukemia (CML)
Translocation of the ABL gene on chromosome 9 to the BCR gene on chromosome 22 creates a chimeric BCR-ABL gene [the Philadelphia chromosome or t(9;22)]. This transcriptionally active gene encodes a fusion protein, which is leukemogenic. We offer both a qualitative test (a multiplex assay which detects p210 and p190 transcripts) as well as quantitative one (especially useful when assessing residual disease following therapeutic interventions). In addition, an ABL kinase domain mutation assay is offered. Mutations in the ABL kinase domain may be associated with resistance to standard-dose treatments for CML, and, without additional therapeutic intervention, with progression to advanced phase disease.
Lymphoma Molecular Testing
Rearrangements of genes that code for antigen receptors occur early in both T-cell and B-cell ontogeny and are unique to each cell. Testing for these rearrangements by PCR is useful for determining whether a clonal population of T cells or B cells is present. We also offer molecular genetic testing that define subsets of B-cell lymphoma including BCL2 testing, and MYD88 analysis. We test for hypermutation of the variable region of the immunoglobulin heavy chain in chronic lymphocytic leukemia (CLL).
Other Testing for Hematolymphoid Malignancies
The Stanford Molecular Pathology laboratory provides testing of SF3B1, JAK2, CALR, and a Cancer Somatic Mutation panel. The acquisition of detailed genomic information in a variety of cancers has demonstrated that a core group of signaling pathways commonly acquire activating mutations. This has led to a paradigm shift in cancer therapy; from knowing the organ of origin and histology as the basis for therapy to a more nuanced approach to additionally specifically target activation mutations which may be common to a variety of cancers. For example activating mutations in BRAF are common in melanoma, lung cancer, thyroid cancer, colorectal cancer and hematologic malignancies. Knowledge of specific mutations may also guide therapeutic decision making to optimally utilize targeted therapies. The mutations detected with this assay are all potentially clinically actionable, typically involving signal pathway activating mutations which have targeted therapies available (some may require enrollment in clinical trials). The SNaPshotTM methodology applied in this test uses a single base extension step with a labeled ddNTP to detect 126 mutations in 12 genes commonly mutated in cancer:

